Abstract
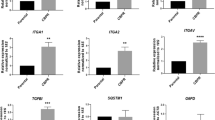
The expression of specificity protein 1 (Sp1) and survivin was evaluated in clinical specimens of epithelial ovarian cancer (EOC) patients. When compared to normal tissue, EOC samples showed high expression of Sp1 and survivin using qPCR (Sp1: ∼2-fold; survivin: ∼5-fold) and Western blot (Sp1: >2.6-fold; survivin: >100-fold). The Sp1 inhibitor, and anti-cancer small molecule, tolfenamic acid (TA), was tested to enhance the response of Cisplatin (Cis) in EOC cell lines. Cell viability (CellTiter-Glo), combination index (CalcuSyn software), apoptosis (Annexin-V staining), cell cycle analyses (flow cytometry), and reactive oxygen species (flow cytometry) were determined. Cell migration and invasion was assessed using matrigel coated transwell chambers. Agilent Technologies proteomics analysis identified potential signaling pathways involved. The combination of TA (50 μM) and Cis (5 μM) synergistically increased the growth inhibition in ES2 (∼80 %, p < 0.001) and OVCAR-3 (60 %, p < 0.001) cells. TA or TA + Cis treatment in ES2 cells caused cell cycle arrest in G1 Phase (TA) or S-Phase (TA + Cis) and unregulated reactive oxygen species. Invasion and migration was decreased in ES2 cells. Global proteomic profiling showed modulation of proteins associated with oxidative phosphorylation, apoptosis, electron transport chain, DNA damage, and cell cycle proteins. These results demonstrate an association of Sp1 and survivin in EOC and confirm targeting these candidates with TA potentially sensitizes EOC cells to cisplatin.








Similar content being viewed by others
References
Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66:7–30.
Ozols RF, Bundy BN, Greer BE, Fowler JM, Clarke-Pearson D, Burger RA, et al. Phase iii trial of carboplatin and paclitaxel compared with cisplatin and paclitaxel in patients with optimally resected stage iii ovarian cancer: a gynecologic oncology group study. J Clin Oncol. 2003;21:3194–200.
Decker DG, Fleming TR, Malkasian Jr GD, Webb MJ, Jeffries JA, Edmonson JH. Cyclophosphamide plus cis-platinum in combination: treatment program for stage III or IV ovarian carcinoma. Obstet Gynecol. 1982;60:481–7.
McGuire WP, Hoskins WJ, Brady MF, Kucera PR, Partridge EE, Look KY, et al. Cyclophosphamide and cisplatin compared with paclitaxel and cisplatin in patients with stage III and stage IV ovarian cancer. N Engl J Med. 1996;334:1–6.
Basha R, Ingersoll SB, Sankpal UT, Ahmad S, Baker CH, Edwards JR, et al. Tolfenamic acid inhibits ovarian cancer cell growth and decreases the expression of c-met and survivin through suppressing specificity protein transcription factors. Gynecol Oncol. 2011;122:163–70.
Abdelrahim M, Baker CH, Abbruzzese JL, Safe S. Tolfenamic acid and pancreatic cancer growth, angiogenesis, and sp protein degradation. J Natl Cancer Inst. 2006;98:855–68.
Jiang NY, Woda BA, Banner BF, Whalen GF, Dresser KA, Lu D. Sp1, a new biomarker that identifies a subset of aggressive pancreatic ductal adenocarcinoma. Cancer Epidemiol Biomarkers Prev. 2008;17:1648–52.
Wang L, Wei D, Huang S, Peng Z, Le X, Wu TT, et al. Transcription factor sp1 expression is a significant predictor of survival in human gastric cancer. Clin Cancer Res. 2003;9:6371–80.
Guan H, Cai J, Zhang N, Wu J, Yuan J, Li J, et al. Sp1 is upregulated in human glioma, promotes mmp-2-mediated cell invasion and predicts poor clinical outcome. Int J Cancer. 2012;130:593–601.
Basha R, Connelly SF, Sankpal UT, Nagaraju GP, Patel H, Vishwanatha JK, et al. Small molecule tolfenamic acid and dietary spice curcumin treatment enhances antiproliferative effect in pancreatic cancer cells via suppressing sp1, disrupting nf-kb translocation to nucleus and cell cycle phase distribution. J Nutr Biochem. 2016;31:77–87.
Sankpal UT, Nagaraju GP, Gottipolu SR, Hurtado M, Jordan CG, Simecka JW, et al. Combination of tolfenamic acid and curcumin induces colon cancer cell growth inhibition through modulating specific transcription factors and reactive oxygen species. OncoTarget. 2016;7:3186–200.
Chou TC. Drug combination studies and their synergy quantification using the chou-talalay method. Cancer Res. 2010;70:440–6.
Chou TC, Talalay P. Quantitative analysis of dose-effect relationships: the combined effects of multiple drugs or enzyme inhibitors. Adv Enzyme Regul. 1984;22:27–55.
Sankpal UT, Abdelrahim M, Connelly SF, Lee CM, Madero-Visbal R, Colon J, et al. Small molecule tolfenamic acid inhibits pc-3 cell proliferation and invasion in vitro, and tumor growth in orthotopic mouse model for prostate cancer. Prostate. 2012;72:1648–58.
He G, Kuang J, Khokhar AR, Siddik ZH. The impact of s- and g2-checkpoint response on the fidelity of g1-arrest by cisplatin and its comparison to a non-cross-resistant platinum(iv) analog. Gynecol Oncol. 2011;122:402–9.
Mattingly RR. Mitogen-activated protein kinase signaling in drug-resistant neuroblastoma cells. Methods Mol Biol. 2003;218:71–83.
Pritchard AL, Hayward NK. Molecular pathways: mitogen-activated protein kinase pathway mutations and drug resistance. Clin Cancer Res. 2013;19:2301–9.
Peng H, Peng T, Wen J, Engler DA, Matsunami RK, Su J, et al. Characterization of p38 mapk isoforms for drug resistance study using systems biology approach. Bioinformatics. 2014;30:1899–907.
Balkwill F, Coussens LM. Cancer: an inflammatory link. Nature. 2004;431:405–6.
Safe S, Abdelrahim M. Sp transcription factor family and its role in cancer. Eur J Cancer. 2005;41:2438–48.
Shi Q, Le X, Abbruzzese JL, Peng Z, Qian CN, Tang H, et al. Constitutive sp1 activity is essential for differential constitutive expression of vascular endothelial growth factor in human pancreatic adenocarcinoma. Cancer Res. 2001;61:4143–54.
Yao JC, Wang L, Wei D, Gong W, Hassan M, Wu TT, et al. Association between expression of transcription factor sp1 and increased vascular endothelial growth factor expression, advanced stage, and poor survival in patients with resected gastric cancer. Clin Cancer Res. 2004;10:4109–17.
Lou Z, O’Reilly S, Liang H, Maher VM, Sleight SD, McCormick JJ. Down-regulation of overexpressed sp1 protein in human fibrosarcoma cell lines inhibits tumor formation. Cancer Res. 2005;65:1007–17.
Colon J, Basha MR, Madero-Visbal R, Konduri S, Baker CH, Herrera LJ, et al. Tolfenamic acid decreases c-met expression through sp proteins degradation and inhibits lung cancer cells growth and tumor formation in orthotopic mice. Investig New Drugs. 2011;29:41–51.
Pennati M, Folini M, Zaffaroni N. Targeting survivin in cancer therapy: fulfilled promises and open questions. Carcinogenesis. 2007;28:1133–9.
Chen L, Liang L, Yan X, Liu N, Gong L, Pan S, et al. Survivin status affects prognosis and chemosensitivity in epithelial ovarian cancer. Int J Gynecol Cancer. 2013;23:256–63.
Cohen C, Lohmann CM, Cotsonis G, Lawson D, Santoianni R. Survivin expression in ovarian carcinoma: correlation with apoptotic markers and prognosis. Mod Pathol. 2003;16:574–83.
Mir R, Stanzani E, Martinez-Soler F, Villanueva A, Vidal A, Condom E, et al. Ym155 sensitizes ovarian cancer cells to cisplatin inducing apoptosis and tumor regression. Gynecol Oncol. 2014;132:211–20.
Pang Y, Mao H, Shen L, Zhao Z, Liu R, Liu P. Mir-519d represses ovarian cancer cell proliferation and enhances cisplatin-mediated cytotoxicity in vitro by targeting xiap. OncoTargets Ther. 2014;7:587–97.
Wagner JM, Karnitz LM. Cisplatin-induced DNA damage activates replication checkpoint signaling components that differentially affect tumor cell survival. Mol Pharmacol. 2009;76:208–14.
Andrews PA, Velury S, Mann SC, Howell SB. Cis-diamminedichloroplatinum(ii) accumulation in sensitive and resistant human ovarian carcinoma cells. Cancer Res. 1988;48:68–73.
Godwin AK, Meister A, O’Dwyer PJ, Huang CS, Hamilton TC, Anderson ME. High resistance to cisplatin in human ovarian cancer cell lines is associated with marked increase of glutathione synthesis. Proc Natl Acad Sci U S A. 1992;89:3070–4.
Kelley SL, Basu A, Teicher BA, Hacker MP, Hamer DH, Lazo JS. Overexpression of metallothionein confers resistance to anticancer drugs. Science. 1988;241:1813–5.
Parker RJ, Eastman A, Bostick-Bruton F, Reed E. Acquired cisplatin resistance in human ovarian cancer cells is associated with enhanced repair of cisplatin-DNA lesions and reduced drug accumulation. J Clin Invest. 1991;87:772–7.
McCubrey JA, Steelman LS, Abrams SL, Lee JT, Chang F, Bertrand FE, et al. Roles of the RAF/MEK/ERK and PI3K/PTEN/AKT pathways in malignant transformation and drug resistance. Adv Enzyme Regul. 2006;46:249–79.
Brozovic A, Osmak M. Activation of mitogen-activated protein kinases by cisplatin and their role in cisplatin-resistance. Cancer Lett. 2007;251:1–16.
Vasko V, Saji M, Hardy E, Kruhlak M, Larin A, Savchenko V, et al. Akt activation and localisation correlate with tumour invasion and oncogene expression in thyroid cancer. J Med Genet. 2004;41:161–70.
Furuya F, Lu C, Willingham MC, Cheng SY. Inhibition of phosphatidylinositol 3-kinase delays tumor progression and blocks metastatic spread in a mouse model of thyroid cancer. Carcinogenesis. 2007;28:2451–8.
Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–74.
Zhu G, Cai G, Liu Y, Tan H, Yu C, Huang M, et al. Quantitative iTRAQ LC-MS/MS proteomics reveals transcription factor crosstalk and regulatory networks in hypopharyngeal squamous cell carcinoma. J Cancer. 2014;5:525–36.
Wang B, Xu W, Tan M, Xiao Y, Yang H, Xia TS. Integrative genomic analyses of a novel cytokine, interleukin-34 and its potential role in cancer prediction. Int J Mol Med. 2015;35:92–102.
Wegdam W, Argmann CA, Kramer G, Vissers JP, Buist MR, Kenter GG, et al. Label-free LC-MSe in tissue and serum reveals protein networks underlying differences between benign and malignant serous ovarian tumors. PLoS ONE. 2014;9, e108046.
Sankpal UT, Lee CM, Connelly SF, Kayaleh O, Eslin D, Sutphin R, et al. Cellular and organismal toxicity of the anti-cancer small molecule, tolfenamic acid: a pre-clinical evaluation. Cell Physiol Biochem. 2013;32:675–86.
Acknowledgments
This work was partially supported by a grant from the Ovarian Cancer Alliance of Florida (awarded to RB), Institute for Cancer Research and Pre-clinical Services, UNTHSC (RB & JWS), Florida Hospital Gala Endowed Program for Oncologic Research (SBI, SA & RWH). JKV is supported by a grant (1P20 MD006882) from the National Institute on Minority Health and Health Disparities, NIH.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclaimer
The information in this manuscript is based on either the published work or the opinion of the authors. The statements made in this manuscript not necessarily reflect the views of authors’ institutions.
Conflicts of interest
None
Rights and permissions
About this article
Cite this article
Sankpal, U.T., Ingersoll, S.B., Ahmad, S. et al. Association of Sp1 and survivin in epithelial ovarian cancer: Sp1 inhibitor and cisplatin, a novel combination for inhibiting epithelial ovarian cancer cell proliferation. Tumor Biol. 37, 14259–14269 (2016). https://doi.org/10.1007/s13277-016-5290-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13277-016-5290-9