Abstract
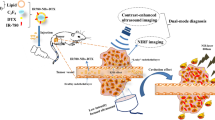
The purpose of this study was to prepare tumor-specific dual-mode nanobubbles as both ultrasound contrast agents (UCAs) and near-infrared fluorescence (NIRF) imaging agents for female tumors. Recent studies have demonstrated the conjugation of anti-tumor ligands on the surface of nanobubbles for use as molecule-targeting ultrasound contrast agents for tumor visualization. However, this complicated procedure has also posed a challenge to nanobubble stability. Thus, in the present study, we combined the fluorescent dye, NIRF IR-780 iodide, which has lipid solubility and tumor-targeting characteristics, with the phospholipid film of nanobubbles that we constructed. We then characterized the physical features of the IR-780-nanobubbles, observed their tumor-targeting capacity in multiple female tumor cell types in vitro, and verified their capability for use in tumor-specific ultrasound contrast imaging and NIRF imaging in vivo. The results showed that the new IR-780-nanobubbles had a uniform nano-size (442.5 ± 48.6 nm) and stability and that they were safe and effective at NIRF imaging and ultrasound imaging in vitro. The IR-780-nanobubbles were found to automatically accumulate on different female tumor cells in vitro with a considerable targeting rate (close to 40 %) but did not accumulate on cardiac muscle cells used as a negative control. Importantly, the IR-780-nanobubbles can detect female tumors precisely via dual-mode imaging in vivo. In conclusion, the new dual-mode IR-780-nanobubbles are stable and have potential advantages in non-invasive tumor-specific detection for female tumors via contrast-enhanced ultrasound and NIRF imaging.








Similar content being viewed by others
References
Nguyen AT, Wrenn SP. Acoustically active liposome-nanobubble complexes for enhanced ultrasonic imaging and ultrasound-triggered drug delivery. Wiley Interdisciplinary Reviews. Nanomedicine Nanobiotechnol. 2014;6:316–25.
Duvshani-Eshet M, Machluf M. Efficient transfection of tumors facilitated by long-term therapeutic ultrasound in combination with contrast agent: from in vitro to in vivo setting. Cancer Gene Ther. 2007;14:306–15.
Huber PE, Pfisterer P. In vitro and in vivo transfection of plasmid DNA in the Dunning prostate tumor R3327-AT1 is enhanced by focused ultrasound. Gene Ther. 2000;7:1516–25.
Mannell H, Pircher J, Rathel T, Schilberg K, Zimmermann K, Pfeifer A, et al. Targeted endothelial gene delivery by ultrasonic destruction of magnetic microbubbles carrying lentiviral vectors. Pharm Res. 2012;29:1282–94.
Anwer K, Kao G, Proctor B, Anscombe I, Florack V, Earls R, et al. Ultrasound enhancement of cationic lipid-mediated gene transfer to primary tumors following systemic administration. Gene Ther. 2000;7:1833–9.
Hosseinkhani H, Tabata Y. Ultrasound enhances in vivo tumor expression of plasmid DNA by PEG-introduced cationized dextran. J Control Release. 2005;108:540–56.
Ferrara KW, Borden MA, Zhang H. Lipid-shelled vehicles: engineering for ultrasound molecular imaging and drug delivery. Acc Chem Res. 2009;42:881–92.
Hobbs SK, Monsky WL, Yuan F, Roberts WG, Griffith L, Torchilin VP, et al. Regulation of transport pathways in tumor vessels: role of tumor type and microenvironment. Proc Natl Acad Sci U S A. 1998;95:4607–12.
Yin T, Wang P, Zheng R, Zheng B, Cheng D, Zhang X, et al. Nanobubbles for enhanced ultrasound imaging of tumors. Int J Nanomedicine. 2012;7:895–904.
Krupka TM, Solorio L, Wilson RE, Wu H, Azar N, Exner AA. Formulation and characterization of echogenic lipid-Pluronic nanobubbles. Mol Pharm. 2010;7:49–59.
Wang Y, Li X, Zhou Y, Huang P, Xu Y. Preparation of nanobubbles for ultrasound imaging and intracelluar drug delivery. Int J Pharm. 2010;384:148–53.
Weller GE, Wong MK, Modzelewski RA, Lu E, Klibanov AL, Wagner WR, et al. Ultrasonic imaging of tumor angiogenesis using contrast microbubbles targeted via the tumor-binding peptide arginine-arginine-leucine. Cancer Res. 2005;65:533–9.
Stieger SM, Dayton PA, Borden MA, Caskey CF, Griffey SM, Wisner ER, et al. Imaging of angiogenesis using Cadence contrast pulse sequencing and targeted contrast agents. Contrast Media Mol Imaging. 2008;3:9–18.
Yang H, Cai W, Xu L, Lv X, Qiao Y, Li P, et al. Nanobubble-affibody: novel ultrasound contrast agents for targeted molecular ultrasound imaging of tumor. Biomaterials. 2015;37:279–88.
Yang X, Shi C, Tong R, Qian W, Zhau HE, Wang R, et al. Near IR heptamethine cyanine dye-mediated cancer imaging. Clin Cancer Res. 2010;16:2833–44.
Bloch M, Jablonowski L, Yavin E, Moradov D, Djavsarov I, Nyska A, et al. Multi-modal detection of colon malignancy by NIR-tagged recognition polymers and ultrasound contrast agents. Int J Pharm. 2015;478:504–16.
Yi X, Wang F, Qin W, Yang X, Yuan J. Near-infrared fluorescent probes in cancer imaging and therapy: an emerging field. Int J Nanomedicine. 2014;9:1347–65.
Ibsen S, Schutt CE, Esener S. Microbubble-mediated ultrasound therapy: a review of its potential in cancer treatment. Drug Des Devel Ther. 2013;7:375–88.
Lanza GM, Abendschein DR, Hall CS, Scott MJ, Scherrer DE, Houseman A, et al. In vivo molecular imaging of stretch-induced tissue factor in carotid arteries with ligand-targeted nanoparticles. J Am Soc Echocardiogr. 2000;13:608–14.
Hughes MS, Marsh JN, Hall CS, Fuhrhop RW, Lacy EK, Lanza GM, et al. Acoustic characterization in whole blood and plasma of site-targeted nanoparticle ultrasound contrast agent for molecular imaging. J Acoust Soc Am. 2005;117:964–72.
Cai WB, Yang HL, Zhang J, Yin JK, Yang YL, Yuan LJ, et al. The optimized fabrication of nanobubbles as ultrasound contrast agents for tumor imaging. Sci Rep. 2015;5:13725.
Chen X, Conti PS, Moats RA. In vivo near-infrared fluorescence imaging of integrin alphavbeta3 in brain tumor xenografts. Cancer Res. 2004;64:8009–14.
Chen Y, Zheng G, Zhang ZH, Blessington D, Zhang M, Li H, et al. Metabolism-enhanced tumor localization by fluorescence imaging: in vivo animal studies. Opt Lett. 2003;28:2070–2.
Graves EE, Weissleder R, Ntziachristos V. Fluorescence molecular imaging of small animal tumor models. Curr Mol Med. 2004;4:419–30.
Moon WK, Lin Y, O’Loughlin T, Tang Y, Kim DE, Weissleder R, et al. Enhanced tumor detection using a folate receptor-targeted near-infrared fluorochrome conjugate. Bioconjug Chem. 2003;14:539–45.
Ntziachristos V, Ripoll J, Wang LV, Weissleder R. Looking and listening to light: the evolution of whole-body photonic imaging. Nat Biotechnol. 2005;23:313–20.
Tung CH, Lin Y, Moon WK, Weissleder R. A receptor-targeted near-infrared fluorescence probe for in vivo tumor imaging. Chembiochem. 2002;3:784–6.
Veiseh M, Gabikian P, Bahrami SB, Veiseh O, Zhang M, Hackman RC, et al. Tumor paint: a chlorotoxin:Cy5.5 bioconjugate for intraoperative visualization of cancer foci. Cancer Res. 2007;67:6882–8.
Gao M, Yu F, Chen H, Chen L. Near-infrared fluorescent probe for imaging mitochondrial hydrogen polysulfides in living cells and in vivo. Anal Chem. 2015;87:3631–8.
Hawrysz DJ, Sevick-Muraca EM. Developments toward diagnostic breast cancer imaging using near-infrared optical measurements and fluorescent contrast agents. Neoplasia. 2000;2:388–417.
Ntziachristos V, Bremer C, Weissleder R. Fluorescence imaging with near-infrared light: new technological advances that enable in vivo molecular imaging. Eur Radiol. 2003;13:195–208.
Yi X, Yan F, Wang F, Qin W, Wu G, Yang X, et al. IR-780 dye for near-infrared fluorescence imaging in prostate cancer. Med Sci Monit. 2015;21:511–7.
Svoboda M, Riha J, Wlcek K, Jaeger W, Thalhammer T. Organic anion transporting polypeptides (OATPs): regulation of expression and function. Curr Drug Metab. 2011;12:139–53.
Shitara Y, Maeda K, Ikejiri K, Yoshida K, Horie T, Sugiyama Y. Clinical significance of organic anion transporting polypeptides (OATPs) in drug disposition: their roles in hepatic clearance and intestinal absorption. Biopharm Drug Dispos. 2013;34:45–78.
James NS, Ohulchanskyy TY, Chen Y, Joshi P, Zheng X, Goswami LN. Comparative tumor imaging and PDT Efficacy of HPPH conjugated in the mono- and di-forms to various polymethine 12cyanine dyes: part - 2. Theranostics. 2013;3:703–18.
Acknowledgments
This work was financially supported by the National Natural Science Foundation of China (grant number 81571730). The authors are grateful to the Department of Pharmaceutical Analysis and the Department of Molecular Biology, Fourth Military Medical University for their technical support and kind provision of equipment.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflicts of interest
None
Additional information
Hengli Yang and Tian Zhou contributed equally to this work.
Rights and permissions
About this article
Cite this article
Yang, H., Zhou, T., Cai, W. et al. Novel dual-mode nanobubbles as potential targeted contrast agents for female tumors exploration. Tumor Biol. 37, 14153–14163 (2016). https://doi.org/10.1007/s13277-016-5238-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13277-016-5238-0