Abstract
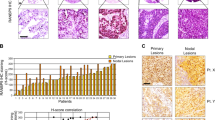
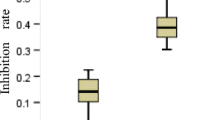
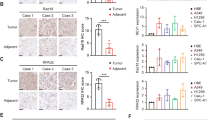
Rad51c is critical for homologous recombination repair and genomic stability and may play roles in tumorigenesis and cancer therapy. We investigated the expression level and clinical significance of Rad51c in non-small cell lung cancer (NSCLC) and determined the effect of Rad51c on NSCLC cell chemosensitivity and radiosensitivity. Rad51c expression was detected using immunohistochemistry and was higher in NSCLC patient samples than in adjacent normal tissues. Kaplan–Meier analysis revealed that high Rad51c expression was an independent predictor of short overall survival (OS) and disease-free survival (DFS) in NSCLC patients receiving chemotherapy and/or radiotherapy. Furthermore, Rad51c knockdown increased the killing effect of ionizing radiation (IR) and enhanced cisplatin-induced apoptotic cells in NSCLC cells by disrupting the repair of cisplatin- and IR-induced DNA damage. In addition, ectopic expression of Rad51c dramatically enhanced NSCLC cell resistance to cisplatin and radiotherapy. These findings suggest that increased expression of Rad51c may confer resistance to chemotherapy and/or radiotherapy of NSCLC, and also be an independent prognostic factor for patient outcome. Therefore, targeting Rad51c may represent an improved therapeutic strategy for NSCLC patients with locally advanced disease.




Similar content being viewed by others
References
Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA Cancer J Clin. 2014;64:9–29.
DeSantis CE, Lin CC, Mariotto AB, Siegel RL, Stein KD, Kramer JL, Alteri R, Robbins AS, Jemal A. Cancer treatment and survivorship statistics, 2014. CA Cancer J Clin. 2014;64:252–71.
Pignon JP, Tribodet H, Scagliotti GV, Douillard JY, Shepherd FA, Stephens RJ, Dunant A, Torri V, Rosell R, Seymour L, Spiro SG, Rolland E, Fossati R, Aubert D, Ding K, Waller D, Le Chevalier T, Group LC. Lung adjuvant cisplatin evaluation: a pooled analysis by the lace collaborative group. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2008;26:3552–9.
Feliciano J, Feigenberg S, Mehta M. Chemoradiation for definitive, preoperative, or postoperative therapy of locally advanced non-small cell lung cancer. Cancer J. 2013;19:222–30.
Jackson SP, Bartek J. The DNA-damage response in human biology and disease. Nature. 2009;461:1071–8.
Kavanagh JN, Redmond KM, Schettino G, Prise KM. DNA double strand break repair: a radiation perspective. Antioxid Redox Signal. 2013;18:2458–72.
Helleday T, Lo J, van Gent DC, Engelward BP. DNA double-strand break repair: from mechanistic understanding to cancer treatment. DNA Repair. 2007;6:923–35.
Aparicio T, Baer R, Gautier J. DNA double-strand break repair pathway choice and cancer. DNA repair. 2014;19:169–75.
Chapman JR, Taylor MR, Boulton SJ. Playing the end game: DNA double-strand break repair pathway choice. Mol Cell. 2012;47:497–510.
Fiume L. Comment on: targeting homologous recombination-mediated DNA repair in cancer. Expert Opin Ther Targets. 2014;18:833.
Helleday T. Homologous recombination in cancer development, treatment and development of drug resistance. Carcinogenesis. 2010;31:955–60.
Birkelbach M, Ferraiolo N, Gheorghiu L, Pfaffle HN, Daly B, Ebright MI, Spencer C, O'Hara C, Whetstine JR, Benes CH, Sequist LV, Zou L, Dahm-Daphi J, Kachnic LA, Willers H: Detection of impaired homologous recombination repair in nsclc cells and tissues. Journal of thoracic oncology : official publication of the International Association for the Study of Lung Cancer 2013;8:279–286.
Cheng J, Liu W, Zeng X, Zhang B, Guo Y, Qiu M, Jiang C, Wang H, Wu Z, Meng M, Zhuang H, Zhao L, Hao J, Cai Q, Xie D, Pang Q, Wang P, Yuan Z, Qian D. Xrcc3 is a promising target to improve the radiotherapy effect of esophageal squamous cell carcinoma. Cancer Sci. 2015
Richardson C. Rad51, genomic stability, and tumorigenesis. Cancer Lett. 2005;218:127–39.
Klein HL. The consequences of rad51 overexpression for normal and tumor cells. DNA repair. 2008;7:686–93.
Nagathihalli NS, Nagaraju G: Rad51 as a potential biomarker and therapeutic target for pancreatic cancer. Biochim Biophys Acta 2011;1816:209–218.
Carvalho JF, Kanaar R. Targeting homologous recombination-mediated DNA repair in cancer. Expert Opin Ther Targets. 2014;18:427–58.
Daley JM, Kwon Y, Niu H, Sung P. Investigations of homologous recombination pathways and their regulation. The Yale Journal of Biology and Medicine. 2013;86:453–61.
Somyajit K, Subramanya S, Nagaraju G. Rad51c: a novel cancer susceptibility gene is linked to fanconi anemia and breast cancer. Carcinogenesis. 2010;31:2031–8.
Liu Y, Masson JY, Shah R, O'Regan P, West SC. Rad51c is required for Holliday junction processing in mammalian cells. Science. 2004;303:243–6.
Badie S, Liao C, Thanasoula M, Barber P, Hill MA, Tarsounas M. Rad51c facilitates checkpoint signaling by promoting chk2 phosphorylation. J Cell Biol. 2009;185:587–600.
Somyajit K, Subramanya S, Nagaraju G. Distinct roles of fanco/rad51c protein in DNA damage signaling and repair: implications for fanconi anemia and breast cancer susceptibility. J Biol Chem. 2012;287:3366–80.
Somyajit K, Saxena S, Babu S, Mishra A, Nagaraju G. Mammalian rad51 paralogs protect nascent DNA at stalled forks and mediate replication restart. Nucleic Acids Res. 2015;43:9835–55.
Li J, Meeks H, Feng BJ, Healey S, Thorne H, Makunin I, Ellis J, kConFab I, Campbell I, Southey M, Mitchell G, Clouston D, Kirk J, Goldgar D, Chenevix-Trench G. Targeted massively parallel sequencing of a panel of putative breast cancer susceptibility genes in a large cohort of multiple-case breast and ovarian cancer families. J Med Genet. 2016;53:34–42.
Couch FJ, Hart SN, Sharma P, Toland AE, Wang X, Miron P, Olson JE, Godwin AK, Pankratz VS, Olswold C, Slettedahl S, Hallberg E, Guidugli L, Davila JI, Beckmann MW, Janni W, Rack B, Ekici AB, Slamon DJ, Konstantopoulou I, Fostira F, Vratimos A, Fountzilas G, Pelttari LM, Tapper WJ, Durcan L, Cross SS, Pilarski R, Shapiro CL, Klemp J, Yao S, Garber J, Cox A, Brauch H, Ambrosone C, Nevanlinna H, Yannoukakos D, Slager SL, Vachon CM, Eccles DM, Fasching PA. Inherited mutations in 17 breast cancer susceptibility genes among a large triple-negative breast cancer cohort unselected for family history of breast cancer. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2015;33:304–11.
Song H, Dicks E, Ramus SJ, Tyrer JP, Intermaggio MP, Hayward J, Edlund CK, Conti D, Harrington P, Fraser L, Philpott S, Anderson C, Rosenthal A, Gentry-Maharaj A, Bowtell DD, Alsop K, Cicek MS, Cunningham JM, Fridley BL, Alsop J, Jimenez-Linan M, Hogdall E, Hogdall CK, Jensen A, Kjaer SK, Lubinski J, Huzarski T, Jakubowska A, Gronwald J, Poblete S, Lele S, Sucheston-Campbell L, Moysich KB, Odunsi K, Goode EL, Menon U, Jacobs IJ, Gayther SA, Pharoah PD. Contribution of germline mutations in the rad51b, rad51c, and rad51d genes to ovarian cancer in the population. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2015;33:2901–7.
Meindl A, Hellebrand H, Wiek C, Erven V, Wappenschmidt B, Niederacher D, Freund M, Lichtner P, Hartmann L, Schaal H, Ramser J, Honisch E, Kubisch C, Wichmann HE, Kast K, Deissler H, Engel C, Muller-Myhsok B, Neveling K, Kiechle M, Mathew CG, Schindler D, Schmutzler RK, Hanenberg H. Germline mutations in breast and ovarian cancer pedigrees establish rad51c as a human cancer susceptibility gene. Nat Genet. 2010;42:410–4.
Osorio A, Endt D, Fernandez F, Eirich K, de la Hoya M, Schmutzler R, Caldes T, Meindl A, Schindler D, Benitez J. Predominance of pathogenic missense variants in the rad51c gene occurring in breast and ovarian cancer families. Hum Mol Genet. 2012;21:2889–98.
Kalvala A, Gao L, Aguila B, Reese T, Otterson GA, Villalona-Calero MA, Duan W. Overexpression of rad51c splice variants in colorectal tumors. Oncotarget. 2015;6:8777–87.
Cai MY, Tong ZT, Zheng F, Liao YJ, Wang Y, Rao HL, Chen YC, QL W, Liu YH, Guan XY, Lin MC, Zeng YX, Kung HF, Xie D. Ezh2 protein: a promising immunomarker for the detection of hepatocellular carcinomas in liver needle biopsies. Gut. 2011;60:967–76.
Ceccaldi R, Liu JC, Amunugama R, Hajdu I, Primack B, Petalcorin MI, O'Connor KW, Konstantinopoulos PA, Elledge SJ, Boulton SJ, Yusufzai T, D'Andrea AD. Homologous-recombination-deficient tumours are dependent on poltheta-mediated repair. Nature. 2015;518:258–62.
Wooster R, Weber BL. Breast and ovarian cancer. N Engl J Med. 2003;348:2339–47.
Werner H, Bruchim I. Igf-1 and brca1 signalling pathways in familial cancer. The Lancet Oncology. 2012;13:e537–44.
Lieberman R, Xiong D, James M, Han Y, Amos CI, Wang L, et al. Functional characterization of rad52 as a lung cancer susceptibility gene in the 12p13.33 locus. Molecular Carcinogenesis. 2015
Qiao GB, YL W, Yang XN, Zhong WZ, Xie D, Guan XY, Fischer D, Kolberg HC, Kruger S, Stuerzbecher HW. High-level expression of rad51 is an independent prognostic marker of survival in non-small-cell lung cancer patients. Br J Cancer. 2005;93:137–43.
Vaz F, Hanenberg H, Schuster B, Barker K, Wiek C, Erven V, Neveling K, Endt D, Kesterton I, Autore F, Fraternali F, Freund M, Hartmann L, Grimwade D, Roberts RG, Schaal H, Mohammed S, Rahman N, Schindler D, Mathew CG. Mutation of the rad51c gene in a fanconi anemia-like disorder. Nat Genet. 2010;42:406–9.
Salama JK, Vokes EE. New radiotherapy and chemoradiotherapy approaches for non-small-cell lung cancer. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2013;31:1029–38.
Takenaka T, Yoshino I, Kouso H, Ohba T, Yohena T, Osoegawa A, Shoji F, Maehara Y. Combined evaluation of rad51 and ercc1 expressions for sensitivity to platinum agents in non-small cell lung cancer. International journal of cancer Journal international du cancer. 2007;121:895–900.
Richardson C, Stark JM, Ommundsen M, Jasin M. Rad51 overexpression promotes alternative double-strand break repair pathways and genome instability. Oncogene. 2004;23:546–53.
Cheng J, Liu W, Zeng X, Zhang B, Guo Y, Qiu M, Jiang C, Wang H, Wu Z, Meng M, Zhuang H, Zhao L, Hao J, Cai Q, Xie D, Pang Q, Wang P, Yuan Z, Qian D. Xrcc3 is a promising target to improve the radiotherapy effect of esophageal squamous cell carcinoma. Cancer Sci. 2015;106:1678–86.
Suwaki N, Klare K, Tarsounas M: Rad51 paralogs: roles in DNA damage signalling, recombinational repair and tumorigenesis. Seminars in cell & developmental biology 2011;22:898–905.
Acknowledgments
This study was supported by Doctor Supporting Foundation of Tianjin Medical University Cancer Institute and Hospital (Nos. B1302 and B1305) and the National Nature Science Foundation of China (Nos. 81401948, 81472182, and 81472797).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflicts of interest
None
Additional information
Xiuli Chen, Dong Qian, and Jingjing Cheng contributed equally to this work.
Rights and permissions
About this article
Cite this article
Chen, X., Qian, D., Cheng, J. et al. High expression of Rad51c predicts poor prognostic outcome and induces cell resistance to cisplatin and radiation in non-small cell lung cancer. Tumor Biol. 37, 13489–13498 (2016). https://doi.org/10.1007/s13277-016-5192-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13277-016-5192-x