Abstract
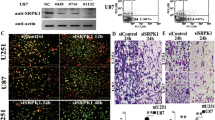
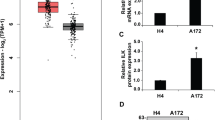
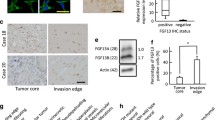
Gliomas are one of the most common primary brain tumors in adults. They display aggressive invasiveness, are highly vascular, and have a poor prognosis. Plexin-B1 is involved in numerous cellular processes, especially cellular migration and angiogenesis. However, the role and regulatory mechanisms of Plexin-B1 in gliomas are not understood and were thus investigated in this study. By using multiple and diverse experimental techniques, we investigated cell apoptosis, mitochondrial membrane potential, cell migration and invasion, angiogenesis, PI3K and Akt phosphorylation, and also the levels of SRPK1 and αvβ3 in glioma cells and animal glioma tissues. The results indicated that Plexin-B1 expression in glioma cell lines is increased compared to normal human astrocytes. Plexin-B1 mediates RhoA/integrin αvβ3 involved in the PI3K/Akt pathway and SRPK1 to influence the growth of glioma cell, angiogenesis, and motility in vitro and in vivo. Thus, Plexin-B1 signaling regulates the Rho/αvβ3/PI3K/Akt pathway and SRPK1, which are involved in glioma invasiveness and angiogenesis. Therefore, the new drug research should focus on Plexin-B1 as a target for the treatment of glioma invasion and angiogenesis.






Similar content being viewed by others
References
Statistics Korea. Statistic of mortality (2014) http://www.nso.go.kr/.
Soderberg-Naucler C, Rahbar A, Stragliotto G. Survival in patients with glioblastoma receiving valganciclovir. N Engl J Med. 2013;369:985–6.
Tamagnone L, Artigiani S, Chen H, He Z, Ming GI, Song H, et al. Plexins are a large family of receptors for transmembrane, secreted, and GPI-anchored semaphorins in vertebrates. Cell. 1999;99:71–80.
Vodrazka P, Korostylev A, Hirschberg A, Swiercz JM, Worzfeld T, Deng S, et al. The semaphorin 4D-plexin-B signalling complex regulates dendritic and axonal complexity in developing neurons via diverse pathways. Eur J Neurosci. 2009;30:1193–208.
Giacobini P, Messina A, Morello F, Ferraris N, Corso S, Penachioni J, et al. Semaphorin 4D regulates gonadotropin hormone-releasing hormone-1 neuronal migration through PlexinB1-Met complex. J Cell Biol. 2008;183:555–66.
Negishi-Koga T, Shinohara M, Komatsu N, Bito H, Kodama T, Friedel RH, et al. Suppression of bone formation by osteoclastic expression of semaphorin 4D. Nat Med. 2011;17:1473–80.
Worzfeld T, Swiercz JM, Looso M, Straub BK, Sivaraj KK, Offermanns S. ErbB-2 signals through Plexin-B1 to promote breast cancer metastasis. J Clin Invest. 2012;122:1296–305.
Zhou H, Binmadi NO, Yang YH, Proia P, Basile JR. Semaphorin 4D cooperates with VEGF to promote angiogenesis and tumor progression. Angiogenesis. 2012;15:391–407.
Zhou Y, Gunput RA, Pasterkamp RJ. Semaphorin signaling: progress made and promises ahead. Trends Biochem Sci. 2008;33:161–70.
Zhang Y, Li Q, Zhuang R, Gao Z, Liu J, Li J, et al. Plexin-B1: a potential diagnostic biomarker for glioma and a future target for glioma immunotherapy. J Neuroimmunol. 2012;252:113–7.
Desgrosellier JS, Cheresh DA. Integrins in cancer: biological implications and therapeutic opportunities. Nat Rev Cancer. 2010;10:9–22.
Kinbara K, Goldfinger LE, Hansen M, Chou FL, Ginsberg MH. Ras GTPases: integrins’ friends or foes? Nat Rev Mol Cell Biol. 2003;4:767–76.
Skuli N, Monferran S, Delmas C, Favre G, Bonnet J, Toulas C, et al. Alphavbeta3/alphavbeta5 integrins-FAK-RhoB: a novel pathway for hypoxia regulation in glioblastoma. Cancer Res. 2009;69:3308–16.
Ellegala DB, Leong-Poi H, Carpenter JE, Klibanov AL, Kaul S, Shaffrey ME, et al. Imaging tumor angiogenesis with contrast ultrasound and microbubbles targeted to alpha(v)beta3. Circulation. 2003;108:336–41.
Zhou B, Li Y, Deng Q, Wang H, Wang Y, Cai B, et al. SRPK1 contributes to malignancy of hepatocellular carcinoma through a possible mechanism involving PI3K/Akt. Mol Cell Biochem. 2013;379:191–9.
Wang P, Zhou Z, Hu A, Ponte de Albuquerque C, Zhou Y, Hong L, et al. Both decreased and increased SRPK1 levels promote cancer by interfering with PHLPP-mediated dephosphorylation of Akt. Mol Cell. 2014;54:378–91.
Wu Q, Chang Y, Zhang L, Zhang Y, Tian T, Feng G, et al. SRPK1 dissimilarly impacts on the growth, metastasis, chemosensitivity and angiogenesis of glioma in normoxic and hypoxic conditions. J Cancer. 2013;4:727–35.
Dussault AA, Pouliot M. Rapid and simple comparison of messenger RNA levels using real-time PCR. Biol Proced Online. 2006;8:1–10.
Hirota K, Semenza GL. Regulation of angiogenesis by hypoxia-inducible factor 1. Crit Rev Oncol Hematol. 2006;59:15–26.
Basile JR, Gavard J, Gutkind JS. Plexin-B1 utilizes RhoA and Rho kinase to promote the integrin-dependent activation of Akt and ERK and endothelial cell motility. J Biol Chem. 2007;282:34888–95.
Bellail AC, Hunter SB, Brat DJ, Tan C, Van Meir EG. Microregional extracellular matrix heterogeneity in brain modulates glioma cell invasion. Int J Biochem Cell Biol. 2004;36:1046–69.
Neufeld G, Shraga-Heled N, Lange T, Guttmann-Raviv N, Herzog Y, Kessler O. Semaphorins in cancer. Front Biosci. 2005;10:751–60.
Goldberg L, Kloog Y. A Ras inhibitor tilts the balance between Rac and Rho and blocks phosphatidylinositol 3-kinase-dependent glioblastoma cell migration. Cancer Res. 2006;66:11709–17.
Wolfenson H, Lavelin I, Geiger B. Dynamic regulation of the structure and functions of integrin adhesions. Dev Cell. 2013;24:447–58.
Ye S, Hao X, Zhou T, Wu M, Wei J, Wang Y, et al. Plexin-B1 silencing inhibits ovarian cancer cell migration and invasion. BMC Cancer. 2010;10:611.
Argast GM, Croy CH, Couts KL, Zhang Z, Litman E, Chan DC, et al. Plexin B1 is repressed by oncogenic B-Raf signaling and functions as a tumor suppressor in melanoma cells. Oncogene. 2009;28:2697–709.
Gomez Roman JJ, Garay GO, Saenz P, Escuredo K, Sanz Ibayondo C, Gutkind S, et al. Plexin B1 is downregulated in renal cell carcinomas and modulates cell growth. Transl Res. 2008;151:134–40.
Amin EM, Oltean S, Hua J, Gammons MV, Hamdollah-Zadeh M, Welsh GI, et al. WT1 mutants reveal SRPK1 to be a downstream angiogenesis target by altering VEGF splicing. Cancer Cell. 2011;20:768–80.
Zhou H, Yang YH, Basile JR. The Semaphorin 4D-Plexin-B1-RhoA signaling axis recruits pericytes and regulates vascular permeability through endothelial production of PDGF-B and ANGPTL4. Angiogenesis. 2014;17:261–74.
Mahabeleshwar GH, Feng W, Reddy K, Plow EF, Byzova TV. Mechanisms of integrin-vascular endothelial growth factor receptor cross-activation in angiogenesis. Circ Res. 2007;101:570–80.
Acknowledgments
This study was supported, in part, by the Key Project of the National Natural Science Foundation of Shandong Province (ZR 2009CL004), the China Postdoctoral Science Foundation (20100481466), the Foundation of Taishan Scholar (tshw20110575), the Pharmaceutical Health Science and Technology Development Program of Shandong Province (2011QZ001, 2013G0021816), the National Natural Science Foundation of China (81171142/H0910, 81271092, 61427807), a Project of the Shandong Province Higher Educational Science and Technology Program (J11LF61), and the Program of Major Research and Development Institutions in Fujian Province (2012I2014).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflicts of interest
None
Additional information
Yingwei Chang and Li Li contributed to this work equally and should be considered as co-first authors
Rights and permissions
About this article
Cite this article
Chang, Y., Li, L., Zhang, L. et al. Plexin-B1 indirectly affects glioma invasiveness and angiogenesis by regulating the RhoA/αvβ3 signaling pathway and SRPK1. Tumor Biol. 37, 11225–11236 (2016). https://doi.org/10.1007/s13277-016-4849-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13277-016-4849-9