Abstract
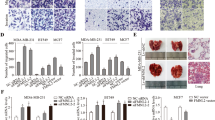
Cell motility and chemotaxis play pivotal roles in the process of tumor development and metastasis. Protein kinase C ζ (PKC ζ) mediates epidermal growth factor (EGF)-stimulated chemotactic signaling pathway through regulating cytoskeleton rearrangement and cell adhesion. The purpose of this study was to develop anti-PKC ζ therapeutics for breast cancer metastasis. In this study, a novel and high-efficient PKC ζ inhibitor named PKCZI195.17 was screened out through a substrate-specific strategy. MTT assay was used to determine the cell viability of human breast cancer MDA-MB-231, MDA-MB-435, and MCF-7 cells while under PKCZI195.17 treatment. Wound-healing, chemotaxis, and Matrigel invasion assays were performed to detect the effects of PKCZI195.17 on breast cancer cells migration and invasion. Adhesion, actin polymerization, and Western blotting were performed to detect the effects of PKCZI195.17 on cells adhesion and actin polymerization, and explore the downsteam signaling mechanisms involved in PKC ζ inhibition. MDA-MB-231 xenograft was used to measure the in vivo anti-metastasis efficacy of PKCZI195.17. The compound PKCZI195.17 selectively inhibited PKC ζ kinase activity since it failed to inhibit PKC α, PKC β, PKC δ, PKC η, AKT2, as well as FGFR2 activity. PKCZI195.17 significantly impaired spontaneous migration, chemotaxis, and invasion of human breast cancer MDA-MB-231, MDA-MB-435, and MCF-7 cells, while PKCZI195.17 did not obviously inhibited cells viability. PKCZI195.17 also inhibited cells adhesion and actin polymerization through attenuating the phosphorylations of integrin β1, LIMK, and cofilin, which might be the downstream effectors of PKC ζ-mediated chemotaxis in MDA-MB-231 cells. Furthermore, PKCZI195.17 suppressed the breast cancer metastasis and increased the survival time of breast tumor-bearing mice. In summary, PKCZI195.17 was a PKC ζ-specific inhibitor which dampened cancer cell migration and metastasis and may serve as a novel therapeutic drug for breast cancer metastasis.





Similar content being viewed by others
References
Thakur S, Singla AK, Chen J, Tran U, Yang Y, Salazar C, et al. Reduced ING1 levels in breast cancer promotes metastasis. Oncotarget. 2014;5(12):4244–56.
Su Z, Yang Z, Xu Y, Chen Y, Yu Q. Apoptosis, autophagy, necroptosis, and cancer metastasis. Mol Cancer. 2015;14:48. doi:10.1186/s12943-015-0321-5.
DeCastro AJ, Cherukuri P, Balboni A, DiRenzo J. ΔNP63α transcriptionally activates chemokine receptor 4 (CXCR4) expression to regulate breast cancer stem cell activity and chemotaxis. Mol Cancer Ther. 2015;14(1):225–35. doi:10.1158/1535-7163.MCT-14-0194.
Dillenburg-Pilla P, Patel V, Mikelis CM, Zárate-Bladés CR, Doçi CL, Amornphimoltham P, et al. SDF-1/CXCL12 induces directional cell migration and spontaneous metastasis via a CXCR4/Gαi/ mTORC1 axis. FASEB J. 2015;29(3):1056–68. doi:10.1096/fj.14-260083.
Feng S, Zhu W. Bidirectional molecular transport shapes cell polarization in a two-dimensional model of eukaryotic chemotaxis. J Theor Biol. 2014;363:235–46. doi:10.1016/j.jtbi.2014.08.033.
Xie L, Lu C, Wu XL. Marine bacterial chemoresponse to a stepwise chemoattractant stimulus. Biophys J. 2015;108(3):766–74. doi:10.1016/j.bpj.2014.11.3479.
Riahi R, Yang YL, Kim H, Jiang L, Wong PK, Zohar Y. A microfluidic model for organ-specific extravasation of circulating tumor cells. Biomicrofluidics. 2014;8(2):024103. doi:10.1063/1.4868301.eCollection2014.
Wang F, Lin SL. Knockdown of kinesin KIF11 abrogates directed migration in response to epidermal growth factor-mediated chemotaxis. Biochem Biophys Res Commun. 2014;452(3):642–8. doi:10.1016/j.bbrc.2014.08.136.
Uddin M, Lau LC, Seumois G, Vijayanand P, Staples KJ, Bagmane D, et al. EGF-induced bronchial epithelial cells drive neutrophil chemotactic and anti-apoptotic activity in asthma. PLoS One. 2013;8(9):e72502. doi:10.1371/journal.pone.0072502.eCollection2013.
Ota I, Higashiyama S, Masui T, Yane K, Hosoi H, Matsuura N. Heparin-binding EGF-like growth factor enhances the activity of invasion and metastasis in thyroid cancer cells. Oncol Rep. 2013;30(4):1593–600. doi:10.3892/or.2013.2659.
Seshacharyulu P, Ponnusamy MP, Rachagani S, Lakshmanan I, Haridas D, Yan Y, et al. Targeting EGF-receptor(s)-STAT1 axis attenuates tumor growth and metastasis through downregulation of MUC4 mucin in human pancreatic cancer. Oncotarget. 2015;6(7):5164–81.
Mori N, Ishikawa C, Senba M. Activation of PKC-δ in HTLV-1-infected T cells. Int J Oncol. 2015;46(4):1609–18. doi:10.3892/ijo.2015.2848.
Lorimer IA. Atypical PKCι as target for glioblastoma therapy. Curr Cancer Drug Targets. 2015;15(2):136–44.
Martin-Liberal J, Cameron AJ, Claus J, Judson IR, Parker PJ, Linch M. Targeting protein kinase C in sarcoma. Biochim Biophys Acta. 2014;1846(2):547–59. doi:10.1016/j.bbcan.2014.10.002.
Vorhagen S, Niessen CM. Mammalian aPKC/Par polarity complex mediated regulation of epithelial division orientation and cell fate. Exp Cell Res. 2014;328(2):296–302. doi:10.1016/j.yexcr.2014.08.008.
Chen J, Zhang M. The Par3/Par6/aPKC complex and epithelial cell polarity. Exp Cell Res. 2013;319(10):1357–64. doi:10.1016/j.yexcr.2013.03.021.
Parker PJ, Justilien V, Riou P, Linch M, Fields AP. Atypical protein kinase Cι as a human oncogene and therapeutic target. Biochem Pharmacol. 2014;88(1):1–11. doi:10.1016/j.bcp.2013.10.023.
Iitaka D, Moodley S, Shimizu H, Bai XH, Liu M. PKCδ-iPLA2-PGE2- PPARγ signaling cascade mediates TNF-α induced Claudin 1 expression in human lung carcinoma cells. Cell Signal. 2015;27(3):568–77. doi:10.1016/j.cellsig.2014.12.015.
Zhao X, Rotenberg SA. Phosphorylation of Cdc42 effector protein-4 (CEP4) by protein kinase C promotes motility of human breast cells. J Biol Chem. 2014;289(37):25844–54. doi:10.1074/jbc.M114.577783.
Liu ZC, Chen XH, Song HX, Wang HS, Zhang G, Wang H, et al. Snail regulated by PKC/GSK-3β pathway is crucial for EGF-induced epithelial-mesenchymal transition (EMT) of cancer cells. Cell Tissue Res. 2014;358(2):491–502. doi:10.1007/s00441-014-1953-2.
Horng CT, Shieh PC, Tan TW, Yang WH, Tang CH. Paeonol suppresses chondrosarcoma metastasis through up-regulation of miR-141 by modulating PKCδ and c-Src signaling pathway. Int J Mol Sci. 2014;15(7):11760–72. doi:10.3390/ijms150711760.
Lee H, Park M, Shin N, Kim G, Kim YG, Shin JS, et al. High mobility group box-1 is phosphorylated by protein kinase C zeta and secreted in colon cancer cells. Biochem Biophys Res Commun. 2012;424(2):321–6. doi:10.1016/j.bbrc.2012.06.116.
Rimessi A, Zecchini E, Siviero R, Giorgi C, Leo S, Rizzuto R, et al. The selective inhibition of nuclear PKCζ restores the effectiveness of chemotherapeutic agents in chemoresistant cells. Cell Cycle. 2012;11(5):1040–8. doi:10.4161/cc.11.5.19520.
Wu J, Zhang B, Wu M, Li H, Niu R, Ying G, et al. Screening of a PKC zeta-specific kinase inhibitor PKCzI257.3 which inhibits EGF-induced breast cancer cell chemotaxis. Invest New Drugs. 2010;28(3):268–75. doi:10.1007/s10637-009-9242-8.
Giagulli C, Scarpini E, Ottoboni L, Narumiya S, Butcher EC, Constantin G, et al. RhoA and zeta PKC control distinct modalities of LFA-1 activation by chemokines: critical role of LFA-1 affinity triggering in lymphocyte in vivo homing. Immunity. 2004;20:25–35. doi:10.1016/S1074-7613(03)00350-9.
Laudanna C, Mochly-Rosen D, Liron T, Constantin G, Butcher EC. Evidence of zeta protein kinase C involvement in polymorphonuclear neutrophil integrin-dependent adhesion and chemotaxis. J Biol Chem. 1998;273:30306–15. doi:10.1074/jbc.273.46.30306.
Guo H, Gu F, Li W, Zhang B, Niu R, Fu L, et al. Reduction of protein kinase C zeta inhibits migration and invasion of human glioblastoma cells. J Neurochem. 2009;109(1):203–13. doi:10.1111/j.1471-4159.2009.05946.x.
Guo H, Ma Y, Zhang B, Sun B, Niu R, Ying G, et al. Pivotal advance: PKCzeta is required for migration of macrophages. J Leukoc Biol. 2009;85(6):911–8. doi:10.1189/jlb.0708429.
Sun R, Gao P, Chen L, Ma D, Wang J, Oppenheim JJ, et al. Protein kinase C zeta is required for epidermal growth factor-induced chemotaxis of human breast cancer cells. Cancer Res. 2005;65:1433–41. doi:10.1158/0008-5472.CAN-04-1163.
Liu Y, Wang B, Wang J, Wan W, Sun R, Zhao Y, et al. Down-regulation of PKC zeta expression inhibits chemotaxis signal transduction in human lung cancer cells. Lung Cancer. 2009;63:210–8. doi:10.1016/j.lungcan.2008.05.010.
Rodems SM, Hamman BD, Lin C, Zhao J, Shah S, Heidary D, et al. A FRET-based assay platform for ultra-high density drug screening of protein kinases and phosphatases. Assay Drug Dev Technol. 2002;1:9–19. doi:10.1089/154065802761001266.
Barati MT, Scherzer J, Wu R, Rane MJ, Klein JB. Cytoskeletal rearrangement and Src and PI-3K-dependent Akt activation control GABA(B)R-mediated chemotaxis. Cell Signal. 2015;27(6):1178–85. doi:10.1016/j.cellsig.2015.02.022.
Reinhardt B, Godfrey R, Fellbrich G, Frank H, Lüske A, Olieslagers S, et al. Human cytomegalovirus infection impairs endothelial cell chemotaxis by disturbing VEGF signalling and actin polymerization. Cardiovasc Res. 2014;104(2):315–25. doi:10.1093/cvr/cvu204.
Wang JN, Wan WZ, Sun RH, Liu Y, Sun XJ, Ma DL, et al. Reduction of Akt2 expression inhibits chemotaxis signal transduction in human breast cancer cells. Cell Signal. 2008;20(6):1025–34. doi:10.1016/j.cellsig.2007.12.023.
Wang JT, Song LZ, Li LL, Zhang W, Chai XJ, An L, et al. Src controls neuronal migration by regulating the activity of FAK and cofilin. Neuroscience. 2015;292:90–100. doi:10.1016/j.neuroscience.2015.02.025.
Wang Y, Kuramitsu Y, Kitagawa T, Baron B, Yoshino S, Maehara SI, et al. Cofilin-phosphatase slingshot-1L (SSH1L) is over-expressed in pancreatic cancer (PC) and contributes to tumor cell migration. Cancer Lett. 2015;360(2):171–6. doi:10.1016/j.canlet.2015.02.015.
Siton O, Bernheim-Groswasser A. Reconstitution of actin-based motility by vasodilator-stimulated phosphoprotein (VASP) depends on the recruitment of F-actin seeds from the solution produced by cofilin. J Biol Chem. 2014;289(45):31274–86. doi:10.1074/jbc.M114.586958.
Li HY, Wu J, Ying GG, Chen LW, Lai LH, Liu Z, et al. J-4: a novel and typical preclinical anticancer drug targeting protein kinase C ζ. Anti- Cancer Drugs. 2012;23(7):691–7.
Huang X, Sun D, Pan Q, Wen WW, Chen Y, Xin XL, et al. JG6, a novel marine-derived oligosaccharide, suppresses breast cancer metastasis via binding to cofilin. Oncotarget. 2014;5(11):3568–78.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (No. 81301985 and No. 81102292) and the Natural Science Foundation of Tianjin (No. 14JCQNJC14000 and No. 12JCQNJC06200; to Jing Wu and Rui Yang).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Compliance with ethical standards
All animal experiments were conducted in accordance with the Guide for the Care and Use of Laboratory Animal of Tianjin Third Central Hospital.
Conflicts of interest
None.
Rights and permissions
About this article
Cite this article
Wu, J., Liu, S., Fan, Z. et al. A novel and selective inhibitor of PKC ζ potently inhibits human breast cancer metastasis in vitro and in mice. Tumor Biol. 37, 8391–8401 (2016). https://doi.org/10.1007/s13277-015-4744-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13277-015-4744-9