Abstract
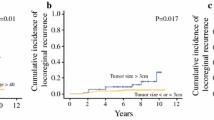
The aim of this study was to examine the association between molecular subtype (MST) and prognosis and research the postmastectomy radiotherapy (PMRT) effect in T1–T2 tumors with 1–3 positive axillary lymph nodes (ALNs). This retrospective study studied breast cancer patients with T1–T2 tumors and 1–3 positive ALNs according to MST: Luminal A, Luminal B, human epidermal growth factor receptor-2 (Her-2) positive, and Triple negative. The impact of adjuvant PMRT in T1–T2 tumors with 1–3 positive ALNs was also assessed. This study included 1369 patients: 33.0 % Luminal A, 42.9 % Luminal B, 11.9 % Her-2 positive, and 12.2 % Triple negative. On univariate and multivariate analyses, MST was associated with locoregional relapse (LRR). Kaplan–Meier analysis showed that PMRT significantly decreased LRR risk (p = 0.017) and distant metastasis (DM) risk (p < 0.0001). In subgroup analysis, PMRT showed significant benefits of improvement in LRR in patients with younger age, positive lymphovascular invasion (LVI), and ratio of positive lymph nodes (LNs) >25 %. Moreover, the nomogram could more accurately predict LRR (c-index 0.75) in T1–2N1 breast cancer patients. MST associated with patient outcomes in breast cancer patients with T1–T2 tumors and 1–3 positive ALN. It makes sense to offer PMRT for patients aged<40 years old, LVI, 2 and 3 positive lymph nodes, and ratio of positive LNs >25 %.



Similar content being viewed by others
References
Wang S, Li W, Liu N, et al. Clinicopathologic characteristics and prognosis for molecular subtypes in low-grade breast carcinoma: comparison with grade one invasive ductal carcinoma-not otherwise specified. Med Oncol. 2012;29:2556–64.
Moo TA, McMillan R, Lee M, et al. Impact of molecular subtype on locoregional recurrence in mastectomy patients with T1–T2 breast cancer and 1–3 positive lymph nodes. Ann Surg Oncol. 2014;21:1569–74.
Fisher B, Redmond C, Fisher ER. The contribution of recent NSABP clinical trials of primary breast cancer therapy to an understanding of tumor biology—an overview of findings. Cancer. 1980;46:1009–25.
Recht A, Edge SB, Solin LJ, et al. Postmastectomy radiotherapy: clinical practice guidelines of the American Society of Clinical Oncology. J Clin Oncol. 2001;19:1539–69.
Shah C, Kundu N, Arthur D, et al. Radiation therapy following postmastectomy reconstruction: a systematic review. Ann Surg Oncol. 2013;20:1313–22.
Whelan TJ, Julian J, Wright J, et al. Does locoregional radiation therapy improve survival in breast cancer? A meta-analysis. J Clin Oncol. 2000;18:1220–9.
Li Y, Moran MS, Huo Q, et al. Post-mastectomy radiotherapy for breast cancer patients with T1–T2 and 1–3 positive lymph nodes: a meta-analysis. PLoS One. 2013;8:e81765.
Sharma R, Bedrosian I, Lucci A, et al. Present-day locoregional control in patients with t1 or t2 breast cancer with 0 and 1 to 3 positive lymph nodes after mastectomy without radiotherapy. Ann Surg Oncol. 2010;17:2899–908.
Tendulkar RD, Rehman S, Shukla ME, et al. Impact of postmastectomy radiation on locoregional recurrence in breast cancer patients with 1–3 positive lymph nodes treated with modern systemic therapy. Int J Radiat Oncol Biol Phys. 2012;83:e577–581.
Jagsi R, Pierce L. Postmastectomy radiation therapy for patients with locally advanced breast cancer. Semin Radiat Oncol. 2009;19:236–43.
Wallgren A, Bonetti M, Gelber RD, et al. Risk factors for locoregional recurrence among breast cancer patients: results from International Breast Cancer Study Group Trials I through VII. J Clin Oncol. 2003;21:1205–13.
EBCTCG (Early Breast Cancer Trialists’ Collaborative Group), McGale P, Taylor C, et al. Effect of radiotherapy after mastectomy and axillary surgery on 10-year recurrence and 20-year breast cancer mortality: meta-analysis of individual patient data for 8135 women in 22 randomised trials. Lancet. 2014;383:2127–35.
Hammond ME, Hayes DF, Dowsett M. American Society of Clinical Oncology/College of American Pathologists guideline recommendations for immunohistochemical testing of estrogen and progesterone receptors in breast cancer. J Clin Oncol. 2010;28:2784–95.
Bianchi S, Caini S, Paglierani M et al. Accuracy and reproducibility of HER2 status in breast cancer using immunohistochemistry: A quality control study in Tuscany evaluating the impact of updated 2013 ASCO/CAP recommendations. Pathol Oncol Res 2014 [Epub ahead of print]
Untch M, Gerber B, Harbeck N, et al. 13th St. Gallen International Breast Cancer Conference 2013: primary therapy of early breast cancer evidence, controversies, consensus—opinion of a German team of experts (Zurich 2013). Breast Care (Basel). 2013;8:221–9.
Kobayashi T, Iwaya K, Moriya T, et al. A simple immunohistochemical panel comprising 2 conventional markers, Ki67 and p53, is a powerful tool for predicting patient outcome in luminal-type breast cancer. BMC Clin Pathol. 2013;13:5.
Hudis CA, Barlow WE, Costantino JP, et al. Proposal for standardized definitions for efficacy end points in adjuvant breast cancer trials: the STEEP system. J Clin Oncol. 2007;25:2127–32.
Delpech Y, Bashour SI, Lousquy R, et al. Clinical nomogram to predict bone-only metastasis in patients with early breast carcinoma. Br J Cancer. 2015;113:1003–9.
Cancello G, Maisonneuve P, Rotmensz N, et al. Prognosis and adjuvant treatment effects in selected breast cancer subtypes of very young women (<35 years) with operable breast cancer. Ann Oncol. 2010;21:1974–81.
Cancello G, Maisonneuve P, Rotmensz N, et al. Prognosis in women with small (T1mic, T1a, T1b) node-negative operable breast cancer by immunohistochemically selected subtypes. Breast Cancer Res Treat. 2011;127:713–20.
Sanchez-Munoz A, Garcia-Tapiador AM, Martinez-Ortega E, et al. Tumour molecular subtyping according to hormone receptors and HER2 status defines different pathological complete response to neoadjuvant chemotherapy in patients with locally advanced breast cancer. Clin Transl Oncol. 2008;10:646–53.
Dragun AE, Huang B, Gupta S, et al. One decade later: trends and disparities in the application of post-mastectomy radiotherapy since the release of the American Society of Clinical Oncology clinical practice guidelines. Int J Radiat Oncol Biol Phys. 2012;83:e591–596.
Caudle AS, Yu TK, Tucker SL, et al. Local-regional control according to surrogate markers of breast cancer subtypes and response to neoadjuvant chemotherapy in breast cancer patients undergoing breast conserving therapy. Breast Cancer Res. 2012;14:R83.
Romond EH, Perez EA, Bryant J, et al. Trastuzumab plus adjuvant chemotherapy for operable HER2-positive breast cancer. N Engl J Med. 2005;353:1673–84.
Yang PS, Chen CM, Liu MC, et al. Radiotherapy can decrease locoregional recurrence and increase survival in mastectomy patients with T1 to T2 breast cancer and one to three positive nodes with negative estrogen receptor and positive lymphovascular invasion status. Int J Radiat Oncol Biol Phys. 2010;77:516–22.
Kyndi M, Sorensen FB, Knudsen H, et al. Estrogen receptor, progesterone receptor, HER-2, and response to postmastectomy radiotherapy in high-risk breast cancer: the Danish Breast Cancer Cooperative Group. J Clin Oncol. 2008;26(9):1419–26.
Chitapanarux I, Tharavichitkul E, Jakrabhandu S, et al. Real-world outcomes of postmastectomy radiotherapy in breast cancer patients with 1–3 positive lymph nodes: a retrospective study. J Radiat Res. 2014;55:121–8.
Cosar R, Uzal C, Tokatli F, et al. Postmastectomy irradiation in breast in breast cancer patients with T1–2 and 1–3 positive axillary lymph nodes: Is there a role for radiation therapy? Radiat Oncol. 2011;6:28.
Su YL, Li SH, Chen YY, et al. Post-mastectomy radiotherapy benefits subgroups of breast cancer patients with T1-2 tumor and 1–3 axillary lymph node(s) metastasis. Radiol Oncol. 2014;48:314–22.
Deng Q, He B, Liu X, et al. Prognostic value of pre-operative inflammatory response biomarkers in gastric cancer patients and the construction of a predictive model. J Transl Med. 2015;13:66.
Acknowledgments
This work was funded by the National Science Foundation of China (81172532, 81470119).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
None
Ethical approval
This study was approved by the Tianjin Medical University Cancer Institute and Hospital, China and has been performed in accordance with the ethical standards laid down in the 1964 Helsinki declaration and its later amendments.
Additional information
Synopsis
Our study examined the association between MST and prognosis and researched the PMRT effect in T1–T2 tumors with 1–3 positive ALNs. Data showed MST associated with patient outcomes in breast cancer patients with T1–T2 tumors and 1–3 positive ALNs. PMRT significantly decreased LRR and DM risk. Our results suggest that PMRT should be offered to patients aged <40 years old, LVI, 2 and 3 positive lymph nodes, and ratio of positive LNs >25 %. The nomogram could more accurately predict LRR (c-index 0.75) in T1–2N1 breast cancer patients.
Rights and permissions
About this article
Cite this article
Shen, H., Zhao, L., Wang, L. et al. Postmastectomy radiotherapy benefit in Chinese breast cancer patients with T1–T2 tumor and 1–3 positive axillary lymph nodes by molecular subtypes: an analysis of 1369 cases. Tumor Biol. 37, 6465–6475 (2016). https://doi.org/10.1007/s13277-015-4546-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13277-015-4546-0