Abstract
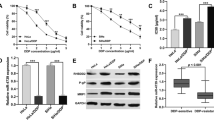
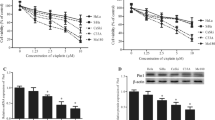
Cervical cancer is one of the most common female malignancies in the world, and chemotherapeutic drug resistance is a major obstacle to cancer therapy. Enhancer of zeste homolog 2 (EZH2) is an enzymatic subunit of polycomb repressive complex 2 (PRC2) and catalyzes the repressive histone H3 lysine 27 trimethylation (H3K27me3). However, the role of EZH2 on the chemotherapy drug resistance in cervical cancers remains unclear. In the present study, the cervical carcinoma specimens and paired normal tissue specimens were obtained and the expression of EZH2 was detected by western blotting. The results showed that high levels of EZH2 were detected in cervical carcinoma tissues, compared with paired control tissues (**p < 0.01). Next, three pairs of shRNA specific to EZH2 were designed and used to interfere with endogenous EZH2 expression. Cell viability was assessed by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assays following treatment with various concentrations of cisplatin in HeLa and HeLa/DDP cells. The MTT assay results showed that knockdown of EZH2 in HeLa/DDP cells caused a 2.29- or 1.83-fold decrease in the cisplatin IC50 values (for shRNA1-EZH2, 34.88 vs. 15.21 μg/mL; p < 0.01; for shRNA3-EZH2, 34.88 vs. 19.09 μg/mL; p < 0.01). The EZH2 activity was also suppressed by 3-deazaneplanocin A (DZNep), EZH2 inhibitor, and the results demonstrated that, meanwhile, DZNep potently inhibited cell viability of HeLa/DDP cells, partly by suppression the levels of EZH2 and H3K27me3, but not H3K27me2, which was detected by western blotting analysis. Moreover, cell migration assay results showed that knockdown of EZH2 decreased cell metastasis of cervical cancer cells. Furthermore, cell cycle was detected by fluorescence-activated cell sorting (FACS) assay and the results demonstrated that interference with EZH2 expression increased the percentage of cells at G0/G1 phase and the HeLa/DDP cells were blocked at G0/G1 phase. Interestingly, western blotting results revealed that higher expression of EZH2 was related with lower level of Dicer in HeLa/DDP cells. Finally, in vivo tumorigenicity experiments results demonstrated that interference with endogenous EZH2 by shRNA specific to EZH2 or inhibition EZH2 by DZNep could significantly increase antitumor effects in nude mice. Thus, inhibiting the levels of endogenous EZH2 effectively reversed the cisplatin resistance and increased the cisplatin sensitivity in cisplatin-resistant HeLa/DDP cells. EZH2 might be a potential target for treating chemotherapeutic drug-resistant cervical cancers.









Similar content being viewed by others
References
Sharma G, Dua P, Agarwal SM. A comprehensive review of dysregulated miRNAs involved in cervical cancer. Curr Genomics. 2014;15:310–23.
Bukowska-Durawa A, Luszczynska A. Cervical cancer screening and psychosocial barriers perceived by patients. A systematic review. Contemp Oncol. 2014;18:153–9.
Touboul C, Skalli D, Guillo E, Martin M, Mallaurie E, Mansouri D, et al. Treatment of cervical cancer. Rev Prat. 2014;64:802–6.
Obel J, Souares Y, Hoy D, Baravilala W, Garland SM, Kjaer SK, et al. A systematic review of cervical cancer incidence and mortality in the Pacific region. Asian Pac J Cancer Prev: APJCP. 2014;15:9433–7.
Banerjee R, Kamrava M. Brachytherapy in the treatment of cervical cancer: a review. Int J Womens Health. 2014;6:555–64.
Tarney CM, Han J. Postcoital bleeding: a review on etiology, diagnosis, and management. Obstet Gynecol Int. 2014;2014:192087.
Fleming S, Schluterman NH, Tracy JK, Temkin SM. Black and white women in maryland receive different treatment for cervical cancer. PLoS One. 2014;9:e104344.
Al-Badawi IA, Al-Suwaine A, Al-Aker M, Asaad L, Alaidan A, Tulbah A, et al. Detection and genotyping of human papilloma virus in cervical cancer specimens from Saudi patients. Int J Gynecol Cancer: Off J Int J Gynecol Cancer Soc. 2011;21:907–10.
Adejuyigbe FF, Balogun BR, Sekoni AO, Adegbola AA. Cervical cancer and human papilloma virus knowledge and acceptance of vaccination among medical students in southwest Nigeria. Afr J Reprod Health. 2015;19:140–8.
Garner D. Clinical application of DNA ploidy to cervical cancer screening: a review. World J Clin Oncol. 2014;5:931–65.
Wang Q, Li YN, Zhai HX, Zhou ZQ, Jia QQ, Ma JW, et al. Clinical significance of detection of human papilloma virus infection with microarray from paraffin-embedded specimens of cervical cancer. Zhonghua bing li xue za zhi Chin J Pathol. 2012;41:842–3.
Yang Y, Ren J, Zhang Q. Distribution of human papilloma virus type 16 e6/e7 gene mutation in cervical precancer or cancer: a case control study in Guizhou province, China. J Med Virol. 2015. doi:10.1002/jmv.24333.
Kokka F, Bryant A, Brockbank E, Powell M, Oram D. Hysterectomy with radiotherapy or chemotherapy or both for women with locally advanced cervical cancer. Cochrane Database Syst Rev. 2015;4:CD010260.
Angioli R, Plotti F, Aloisi A, Scaletta G, Capriglione S, Luvero D, et al. A randomized controlled trial comparing four versus six courses of adjuvant platinum-based chemotherapy in locally advanced cervical cancer patients previously treated with neo-adjuvant chemotherapy plus radical surgery. Gynecol Oncol. 2015. doi:10.1016/j.ygyno.2015.09.082.
Eskander RN, Tewari KS. Chemotherapy in the treatment of metastatic, persistent, and recurrent cervical cancer. Curr Opin Obstet Gynecol. 2014;26:314–21.
Zhang G, Liu F, Jia E, Jia L, Zhang Y. Folate-modified, cisplatin-loaded lipid carriers for cervical cancer chemotherapy. Drug Deliv. 2015:1–5.
Crescencio ME, Rodriguez E, Paez A, Masso FA, Montano LF, Lopez-Marure R. Statins inhibit the proliferation and induce cell death of human papilloma virus positive and negative cervical cancer cells. Int J Biomed Sci: IJBS. 2009;5:411–20.
Deodhar KK. Screening for cervical cancer and human papilloma virus: Indian context. Clin Lab Med. 2012;32:193–205.
Liu Y, Liu T, Bao X, He M, Li L, Yang X. Increased ezh2 expression is associated with proliferation and progression of cervical cancer and indicates a poor prognosis. Int J Gynecol Pathol: Off J Int Soc Gynecol Pathol. 2014;33:218–24.
Lin WC, Yan MD, Yu PN, Li HJ, Kuo CC, Hsu CL, et al. The role of sp1 and ezh2 in the regulation of lmx1a in cervical cancer cells. Biochim Biophys Acta. 1833;2013:3206–17.
Sun NX, Ye C, Zhao Q, Zhang Q, Xu C, Wang SB, et al. Long noncoding RNA-EBIC promotes tumor cell invasion by binding to EZH2 and repressing E-cadherin in cervical cancer. PLoS One. 2014;9:e100340.
Hubaux R, Thu KL, Coe BP, MacAulay C, Lam S, Lam WL. EZH2 promotes E2f-driven SCLC tumorigenesis through modulation of apoptosis and cell-cycle regulation. J Thorac Oncol: Off Publ Int Assoc Study of Lung Cancer. 2013;8:1102–6.
Mattioli E, Vogiatzi P, Sun A, Abbadessa G, Angeloni G, D’Ugo D, et al. Immunohistochemical analysis of pRb2/p130, VEGF, EZH2, p53, p16(INK4A), p27(KIP1), p21(WAF1), Ki-67 expression patterns in gastric cancer. J Cell Physiol. 2007;210:183–91.
Seward S, Semaan A, Qazi AM, Gruzdyn OV, Chamala S, Bryant CC, et al. EZH2 blockade by RNA interference inhibits growth of ovarian cancer by facilitating re-expression of p21(waf1/cip1) and by inhibiting mutant p53. Cancer Lett. 2013;336:53–60.
Han Li C, Chen Y. Targeting EZH2 for cancer therapy: progress and perspective. Curr Protein Pept Sci. 2015;16:559–70.
Kim W, Bird GH, Neff T, Guo G, Kerenyi MA, Walensky LD, et al. Targeted disruption of the EZH2-EED complex inhibits EZH2-dependent cancer. Nat Chem Biol. 2013;9:643–50.
Li LY. EZH2: novel therapeutic target for human cancer. BioMedicine. 2014;4:1.
McCabe MT, Creasy CL. EZH2 as a potential target in cancer therapy. Epigenomics. 2014;6:341–51.
Yamaguchi H, Hung MC. Regulation and role of EZH2 in cancer. Cancer Res Treat: Off J Korean Cancer Assoc. 2014;46:209–22.
Adhikary G, Grun D, Balasubramanian S, Kerr C, Huang JM, Eckert RL. Survival of skin cancer stem cells requires the Ezh2 polycomb group protein. Carcinogenesis. 2015;36:800–10.
Liu YL, Gao X, Jiang Y, Zhang G, Sun ZC, Cui BB, et al. Expression and clinicopathological significance of EED, SUZ12 and EZH2 mRNA in colorectal cancer. J Cancer Res Clin Oncol. 2015;141:661–9.
Reijm EA, Timmermans AM, Look MP, Meijer-van Gelder ME, Stobbe CK, van Deurzen CH, et al. High protein expression of EZH2 is related to unfavorable outcome to tamoxifen in metastatic breast cancer. Ann Oncol: Off J Eur Soc Med Oncol / ESMO. 2014;25:2185–90.
Zhang Y, Liu G, Lin C, Liao G, Tang B. Silencing the EZH2 gene by RNA interference reverses the drug resistance of human hepatic multidrug-resistant cancer cells to 5-FU. Life Sci. 2013;92:896–902.
Xu K, Wu ZJ, Groner AC, He HH, Cai C, Lis RT, et al. Ezh2 oncogenic activity in castration-resistant prostate cancer cells is polycomb-independent. Science. 2012;338:1465–9.
Yoo KH, Hennighausen L. EZH2 methyltransferase and H3K27 methylation in breast cancer. Int J Biol Sci. 2012;8:59–65.
Yu H, Simons DL, Segall I, Carcamo-Cavazos V, Schwartz EJ, Yan N, et al. PRC2/EED-EZH2 complex is up-regulated in breast cancer lymph node metastasis compared to primary tumor and correlates with tumor proliferation in situ. PLoS One. 2012;7:e51239.
Li K, Liu C, Zhou B, Bi L, Huang H, Lin T, et al. Role of EZH2 in the growth of prostate cancer stem cells isolated from LNCaP cells. Int J Mol Sci. 2013;14:11981–93.
Bryant RJ, Cross NA, Eaton CL, Hamdy FC, Cunliffe VT. EZH2 promotes proliferation and invasiveness of prostate cancer cells. Prostate. 2007;67:547–56.
Xie L, Zhang Z, Tan Z, He R, Zeng X, Xie Y, et al. MicroRNA-124 inhibits proliferation and induces apoptosis by directly repressing EZH2 in gastric cancer. Mol Cell Biochem. 2014;392:153–9.
He LJ, Cai MY, Xu GL, Li JJ, Weng ZJ, Xu DZ, et al. Prognostic significance of overexpression of EZH2 and H3k27me3 proteins in gastric cancer. Asian Pac J Cancer Prev: APJCP. 2012;13:3173–8.
Zhou W, Wang J, Man WY, Zhang QW, Xu WG. SiRNA silencing EZH2 reverses cisplatin-resistance of human non-small cell lung and gastric cancer cells. Asian Pac J Cancer Prev: APJCP. 2015;16:2425–30.
Xia H, Zhang W, Li Y, Guo N, Yu C. Ezh2 silencing with rna interference induces g2/m arrest in human lung cancer cells in vitro. BioMed Res Int. 2014;2014:348728.
Geng J, Li X, Zhou Z, Wu CL, Dai M, Bai X. EZH2 promotes tumor progression via regulating VEGF-A/Akt signaling in non-small cell lung cancer. Cancer Lett. 2015;359:275–87.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Cai, L., Wang, Z. & Liu, D. Interference with endogenous EZH2 reverses the chemotherapy drug resistance in cervical cancer cells partly by up-regulating Dicer expression. Tumor Biol. 37, 6359–6369 (2016). https://doi.org/10.1007/s13277-015-4416-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13277-015-4416-9