Abstract
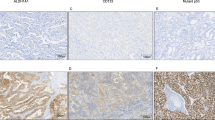
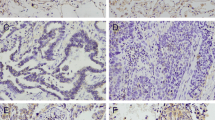
We aimed to study the expression status of β-arrestin1 in non-small cell lung cancer (NSCLC) specimens and its clinicopathologic significance. The correlation between β-arrestin1 and the tumor migration biomarker E-cadherin, as well as smoking index were studied. A total of 152 patients with NSCLC who undergone surgery were enrolled. Altogether, 88 lung squamous cell lung cancer (SCC) specimens and 64 adenocarcinoma (ADC) specimens were tested for immunohistochemistry. Patients’ survival was analyzed by the Kaplan–Meier method. Univariate and multivariate analyses were performed to determine independent prognostic factors. Spearman rank correlation test was used to show data associations. For SCC patients, the expression of β-arrestin1 was either lost (56 of 88, 63.6 %) or low (32 of 88, 36.4 %), which was significantly and negatively associated with E-cadherin expression (P = 0.017). The similar correlation existed between smoking index and β-arrestin1 expression (P = 0.044). For ADC patients, the deletion of β-arrestin1 expression was rare (4 of 64, 6.3 %). Loss of β-arrestin1 expression indicated poorer survival for both SCC (P = 0.026) and ADC patients (P = 0.006). β-arrestin1 expression was detected in the other ADC specimens but showed no significant correlation with survival. In SCC patients, the loss expression of β-arrestin1 was frequently observed, and β-arrestin1 expression was significantly correlated with the smoking index and E-cadherin expression, which all indicated β-arrestin1’s significant clinicopathologic role. However, β-arrestin1 was expressed in most ADC patients, but its clinicopathologic role seemed to be obscure and might need further exploration.




Similar content being viewed by others
References
Jemal A, Bray F, Center MM, et al. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90.
Cagle PT, Chirieac LR. Advances in treatment of lung cancer with targeted therapy. Arch Pathol Lab Med. 2012;136:504–9.
Krupnick JG, Benovic JL. The role of receptor kinases and arrestins in G protein-coupled receptor regulation. Annu Rev Pharmacol Toxicol. 1998;38:289–319.
Moore CA, Milano SK, Benovic JL. Regulation of receptor trafficking by GRKs and arrestins. Annu Rev Physiol. 2007;69:451–82.
Buchanan FG, DuBois RN. Emerging roles of β-arrestins. Cell Cycle. 2006;5:2060–3.
Kovacs JJ, Hara MR, Davenport CL, et al. Arrestin development: emerging roles for beta arrestins in developmental signaling pathways. Dev Cell. 2009;17:443–58.
Ge L, Ly Y, Hollenberg M, DeFea K. Beta-arrestin-dependent scaffold is associated with prolonged MAPK activation in pseudopodia during protease-activated receptor-2-induced chemotaxis. J Biol Chem. 2003;278:34418–26.
Sun Y, Cheng Z, Ma L, et al. Beta-arrestin2 is critically involved in CXCR4-mediated chemotaxis, and this is mediated by its enhancement of p38 MAPK activation. J Biol Chem. 2002;277:49212–9.
Zoudilova M, Kumar P, Ge L, et al. Beta-arrestin-dependent regulation of the cofilin pathway downstream of protease-activated receptor-2. J Biol Chem. 2007;282:20634–46.
Zoudilova M, Min J, Richards HL, et al. Beta-arrestins scaffold cofilin with chronophin to direct localized actin filament severing and membrane protrusions downstream of protease-activated receptor-2. J Biol Chem. 2010;285:14318–29.
Wang P, DeFea KA. Protease-activated receptor-2 simultaneously directs beta-arrestin-1-dependent inhibition and Galphaq-dependent activation of phosphatidylinositol 3-kinase. Biochemistry. 2006;45:9374–85.
Girnita L, Shenoy SK, Sehat B, et al. Beta-arrestin is crucial for ubiquitination and down-regulation of the insulin-like growth factor-1 receptor by acting as adaptor for the MDM2 E3 ligase. J Biol Chem. 2005;280:24412–9.
Shenoy SK, Lefkowitz RJ. β-Arrestin-mediated receptor trafficking and signal transduction. Trends Pharmcol Sci. 2011;32:521–33.
Lakshmikanthan V, Zou L, Kim JI, et al. Identification of beta-arrestin2 as a corepressor of androgen receptor signaling in prostate cancer. Proc Natl Acad Sci USA. 2009;106:9379–84.
Michal AM, Peck AR, Tran TH, et al. Differential expression of arrestins is a predictor of breast cancer progression and survival. Breast Cancer Res Treat. 2011;130:791–807.
Wang LG, Su BH, Du JJ. Expression of β-arrestin1 in gastric cardiac adenocarcinoma and its relation with progression. Asian Pacific J Cancer Prev. 2012;13:5671–5.
Ueda Y, Neel NF, Schutyser E, et al. Deletion of the COOH-terminal domain of CXC chemokine receptor 4 leads to the down-regulation of cell-to-cell contact, enhanced motility and proliferation in breast carcinoma cells. Cancer Res. 2006;66:5665–565.
Rosanò L, Cianfrocca R, Tocci P, et al. β-arrestin-1 is a nuclear transcriptional regulator of endothelin-1-induced β-catenin signaling. Oncogene. 2013;32:5066–77.
Yang JY, Zong CS, Xia W, et al. MDM2 promotes cell motility and invasiveness by regulating E-cadherin degradation. Mol Cell Biol. 2006;26:7269–82.
Lymperopoulos A, Negussie S. β-Arrestins in cardiac G protein-coupled receptor signaling and function: partners in crime or “good cop, bad cop”? Int J Mol Sci. 2013;14:24726–41.
Lymperopoulos A, Bathgate A. Arrestins in the cardiovascular system. Prog Mol Biol Transl Sci. 2013;118:297–334.
Hu S, Wang D, Wu J, et al. Involvement of β-arrestins in cancer progression. Mol Biol Rep. 2013;40:1065–71.
Dasgupta P, Rastogi S, Pillai S, et al. Nicotine induces cell proliferation by beta-arrestin mediated activation of Src and Rb-Raf-1 pathways. J Clin Invest. 2006;116:2208–17.
Dasgupta P, Rizwani W, Pillai S, et al. β-arrestin1 mediated regulation of E2F target genes in nicotine-induced growth of lung tumors. J Natl Cancer Inst. 2011;103:317–33.
Saunders W. Centrosomal amplification and spindle multipolarity in cancer cells. Semin Cancer Biol. 2005;15:25–32.
Doxsey S, Zimmerman W, Mikule K. Centrosome control of the cell cycle. Trends Cell Biol. 2005;15:303–11.
Molla-Herman A, Boularan C, Ghossoub R, et al. Targeting of beta-arrestin2 to the centrosome and primary cilium: role in cell proliferation control. PLoS ONE. 2008;3:e3728.
Shankar H, Michal A, Kern RC, et al. Non-visual arrestins are constitutively associated with the centrosome and regulate centrosome function. J Biol Chem. 2010;285:8316–9.
Perumal D, Pillai S, Nguyen J, et al. Nicotinic acetylcholine receptors induce c-Kit ligand/stem cell factor and promote stemness in an ARRB1/β-arrestin-1 dependent manner in NSCLC. Oncotarget. 2014;5:10486–502.
Acknowledgments
The authors thank doctors Lin and Liu of the Department of Pathology, Shandong Provincial Hospital Affiliated to Shandong University, for their assistance in the processing of tissue sections and the assessment of immunostaining results. This work was supported by Provincial Science and Technology Development Planning of Shandong (2012G0021836), Shandong Provincial Natural Science Foundation of China (ZR2013HZ001), and National Natural Science Foundation of China (81301728).
Compliance with ethical standards
ᅟ
Conflicts of interest
None
Research involving human participants and/or animals
Our research was approved by Ethical Committee of Shandong Provincial Hospital affiliated to Shandong University.
Informed consent
The informed written consent for the use of their clinical study was obtained from every investigated patients.
Author information
Authors and Affiliations
Corresponding author
Additional information
Honghai Ma and Liguang Wang contributed equally to this work.
Rights and permissions
About this article
Cite this article
Ma, H., Wang, L., Zhang, T. et al. Loss of β-arrestin1 expression predicts unfavorable prognosis for non-small cell lung cancer patients. Tumor Biol. 37, 1341–1347 (2016). https://doi.org/10.1007/s13277-015-3886-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13277-015-3886-0