Abstract
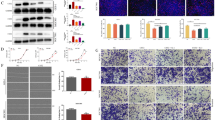
Ubiquitin C-terminal hydrolase-L1 (UCHL1) is a de-ubiquitinating enzyme, which enzymatic activity relies on the C90 site. The function of UCHL1 is controversial in different types of cancer, and its role in gastric cancer progression remains unclear. In this study, immunohistochemistry staining was applied to detect the expression of UCHL1 in primary gastric cancer and liver metastases from gastric cancer. MKN45 and BGC823 cell lines with stable expression of de-ubiquitinase active UCHL1 or inactive UCHL1-variant C90S were established by lentiviral infection. The effect of UCHL1 on cell proliferation was evaluated by MTT and colony formation assays. The abilities of cell migration and invasion were determined by transwell assay. Protein expression levels were determined by Western blot. The results indicated that UCHL1 had a significantly higher positive expression rate in liver metastases from gastric cancer compared with primary gastric cancer. Overexpression of UCHL1 in MKN45 and BGC823 cells promoted cell proliferation, migration, and invasion depending on its de-ubiquitinase activity. UCHL1 activated Akt and Erk1/2, which process also required enzymatic activity and was necessary for mediating cell migration and invasion. These findings demonstrated that UCHL1 promoted cell proliferation, migration, and invasion depending on its de-ubiquitinase activity by activating Akt and Erk1/2, which may account for its higher positive expression rate in liver metastases from gastric cancer. UCHL1 could be a candidate biomarker and a therapeutic target for gastric cancer metastasis.





Similar content being viewed by others
References
Park JY, von Karsa L, Herrero R. Prevention strategies for gastric cancer: a global perspective. Clin Endosc. 2014;47(6):478–89.
Steeg PS. Tumor metastasis: mechanistic insights and clinical challenges. Nat Med. 2006;12(8):895–904.
Kakeji Y, Morita M, Maehara Y. Strategies for treating liver metastasis from gastric cancer. Surg Today. 2010;40(4):287–94.
Larsen CN, Price JS, Wilkinson KD. Substrate binding and catalysis by ubiquitin C-terminal hydrolases: identification of two active site residues. Biochemistry. 1996;35(21):6735–44.
Yu J, Tao Q, Cheung KF, Jin H, Poon FF, Wang X, et al. Epigenetic identification of Ubiquitin carboxyl-terminal hydrolase L1 as a functional tumor suppressor and biomarker for hepatocellular carcinoma and other digestive tumors. Hepatology. 2008;48(2):508–18.
Xiang T, Li L, Yin X, Yuan C, Tan C, Su X, et al. The ubiquitin peptidase UCHL1 induces G0/G1 cell cycle arrest and apoptosis through stabilizing p53 and is frequently silenced in breast cancer. PLoS One. 2012;7(1):e29783. doi:10.1371/journal.pone.0029783.
Kim HJ, Kim YM, Lim S, Nam YK, Jeong J, Kim H-J, et al. Ubiquitin C-terminal hydrolase-L1 is a key regulator of tumor cell invasion and metastasis. Oncogene. 2009;28(1):117–27.
Hussain S, Foreman O, Perkins SL, Witzig TE, Miles RR, Van Deursen J, et al. The de-ubiquitinase UCH-L1 is an oncogene that drives the development of lymphoma in vivo by deregulating PHLPP1 and Akt signaling. Leukemia. 2010;24(9):1641–55.
Jang MJ, Baek SH, Kim JH. UCH-L1 promotes cancer metastasis in prostate cancer cells through EMT induction. Cancer Lett. 2011;302(2):128–35.
Zhong J, Zhao M, Ma Y, Luo Q, Liu J, Wang J, et al. UCHL1 acts as a colorectal cancer oncogene via activation of the β-catenin/TCF pathway through its deubiquitinating activity. Int J Mol Med. 2012;30(2):430–6.
Wulfanger J, Biehl K, Tetzner A, Wild P, Ikenberg K, Meyer S, et al. Heterogeneous expression and functional relevance of the ubiquitin carboxyl-terminal hydrolase L1 in melanoma. Int J Cancer. 2013;133(11):2522–32.
Zheng S, Qiao G, Min D, Zhang Z, Lin F, Yang Q, et al. Heterogeneous expression and biological function of ubiquitin carboxy-terminal hydrolase-L1 in osteosarcoma. Cancer Lett. 2015;359(1):36–46.
Lien HC, Wang CC, Lin CH, Lu YS, Huang CS, Hsiao LP, et al. Differential expression of ubiquitin carboxy-terminal hydrolase L1 in breast carcinoma and its biological significance. Hum Pathol. 2013;44(9):1838–48.
Mizukami H, Goto T, Kitamura Y, Sakata M, Saito M, Ishibashi K, et al. PGP9.5 was less frequently methylated in advanced gastric carcinoma. Hepatogastroenterology. 2009;56(94–95):1576–9.
Cairns RA, Khokha R, Hill RP. Molecular mechanisms of tumor invasion and metastasis: an integrated view. Curr Mol Med. 2003;3(7):659–71.
Chun J, Kim YS. Platycodin D inhibits migration, invasion, and growth of MDA-MB-231 human breast cancer cells via suppression of EGFR-mediated Akt and MAPK pathways. Chem Biol Interact. 2013;205(3):212–21.
Orditura M, Galizia G, Sforza V, Gambardella V, Fabozzi A, Laterza MM, et al. Treatment of gastric cancer. World J Gastroenterol. 2014;20(7):1635–49.
Polivka Jr J, Janku F. Molecular targets for cancer therapy in the PI3K/AKT/mTOR pathway. Pharmacol Ther. 2014;142(2):164–75.
Dhillon AS, Hagan S, Rath O, Kolch W. MAP kinase signalling pathways in cancer. Oncogene. 2007;26(22):3279–90.
Chin YR, Toker A. Function of Akt/PKB signaling to cell motility, invasion and the tumor stroma in cancer. Cell Signal. 2009;21(4):470–6.
Huang C, Jacobson K, Schaller MD. MAP kinases and cell migration. J Cell Sci. 2004;117:4619–28.
Davis FM, Stewart TA, Thompson EW, Monteith GR. Targeting EMT in cancer: opportunities for pharmacological intervention. Trends Pharmacol Sci. 2014;35(9):479–88.
Grille SJ, Bellacosa A, Upson J, Klein-Szanto AJ, van Roy F, Lee-Kwon W, et al. The protein kinase Akt induces epithelial mesenchymal transition and promotes enhanced motility and invasiveness of squamous cell carcinoma lines. Cancer Res. 2003;63(9):2172–8.
Xie L, Law BK, Chytil AM, Brown KA, Aakre ME, Moses HL. Activation of the Erk pathway is required for TGF-beta1-induced EMT in vitro. Neoplasia. 2004;6(5):603–10.
Elsum IA, Martin C, Humbert PO. Scribble regulates an EMT polarity pathway through modulation of MAPK-ERK signaling to mediate junction formation. J Cell Sci. 2013;126(Pt 17):3990–9.
Bheda A, Shackelford J, Pagano JS. Expression and functional studies of ubiquitin C-terminal hydrolase L1 regulated genes. PLoS One. 2009;4(8), e6764.
Frisan T, Coppotelli G, Dryselius R, Masucci MG. Ubiquitin C-terminal hydrolase-L1 interacts with adhesion complexes and promotes cell migration, survival, and anchorage independent growth. FASEB J. 2012;26(12):5060–70.
Goto Y, Zeng L, Yeom CJ, Zhu Y, Morinibu A, Shinomiya K, et al. UCHL1 provides diagnostic and antimetastatic strategies due to its deubiquitinating effect on HIF-1α. Nat Commun. 2015;6:6153. doi:10.1038/ncomms7153.
Acknowledgments
This study was supported by the National Natural Science Foundation of China (81472208) and the Open Projects of State Key Laboratory of Molecular Oncology (SKL-KF-2015-12). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Conflicts of interest
None
Author information
Authors and Affiliations
Corresponding authors
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOCX 559 kb)
Rights and permissions
About this article
Cite this article
Gu, Yy., Yang, M., Zhao, M. et al. The de-ubiquitinase UCHL1 promotes gastric cancer metastasis via the Akt and Erk1/2 pathways. Tumor Biol. 36, 8379–8387 (2015). https://doi.org/10.1007/s13277-015-3566-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13277-015-3566-0