Abstract
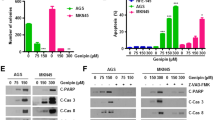
The study aims to investigate the relationship between nuclear factor (nuclear factor kappa B (NF-κB)) viability and lactacystin-mediated cell apoptosis in gastric cancer cells. Two gastric cancer cell lines (MKN28 and SGC7901) were treated with lactacystin—a proteasome inhibitor for 24 h. The cell viability, toxicity, and death were measured by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) method. DNA binding viability of NF-κB and caspase-3 viability were analyzed by ELISA; the expression of p65 NF-κB nuclear protein was detected by immunocytochemistry and Western blot. Lactacystin reduced DNA binding viability of NF-κB (t = 3.0,P = 0.013) and the NF-κB viability (compared to the 5, 10 μmol/L MKN28 cell (p53 mutant) line, P < 0.001) and the expression of p65 NF-κB nuclear protein decreased parallelled to concentrations of lactacystin in MKN28 cell line, while without obvious effects on NF-κB viability in SGC7901 cell line (P = 0.381), while the viability of caspase-3 increased also along with the raising of lactacystin concentrations (compared to control, 5 μmol/L: SGC7901 cell line P = 0.029, MKN28 cell line P < 0.001; 10 μmol/L: SGC7901 cell line, P < 0.001, MKN28 cell line, P < 0.001). It was concluded that lactacystin had diversified killing effects on gastric cancer cells. The mechanism may be related to induce the apoptosis by downregulation of nuclear factor kappa B viability. There may be additional cell survival/death pathway in SGC7901 gastric cancer cells.








Similar content being viewed by others
References
Liu W, Yang Q, Liu B, Zhu Z. Serum proteomics for gastric cancer. Clin Chim Acta. 2014;431:179–84.
Fan XM, Wong BCY, Wang WP, Zhou XM, Cho CH, Yuen ST, et al. Inhibition of proteasome function induced apoptosis in gastric cancer. Int J Cancer. 2001;93:481–8.
Cohen G. Caspases: the executioners of apoptosis. Biochem J. 1997;326:1–16.
Milacic V, Banerjee S, Landis-Piwowar KR, Sarkar FH, Majumdar AP, Dou QP. Curcumin inhibits the proteasome activity in human colon cancer cells in vitro and in vivo. Cancer Res. 2008;68:7283–92.
Cusimano A, Azzolina A, Iovanna JL, Bachvarov D, McCubrey JA, D’Alessandro N, et al. Novel combination of celecoxib and proteasome inhibitor mg132 provides synergistic antiproliferative and proapoptotic effects in human liver tumor cells. Cell Cycle. 2010;9:1399–410.
Rastogi N, Mishra DP. Therapeutic targeting of cancer cell cycle using proteasome inhibitors. Cell Div. 2012;26:7–26.
Slater E, Waldmann J, López C, Matthäi E, Fendrich V, Bartsch D. Pancreatic neuroendocrine tumors are characterized by loss of noxa expression that can be recovered by the proteasome inhibitor bortezomib. J Cancer Ther. 2013;4:28.
Budunova I. Differential targeting of androgen and glucocorticoid receptors induces er stress and apoptosis in prostate cancer cells. Cell Cycle. 2012;11:395–406.
Chen J, Giridhar KV, Zhang L, Xu S, Wang QJ. A protein kinase c/protein kinase d pathway protects lncap prostate cancer cells from phorbol ester-induced apoptosis by promoting erk1/2 and nf-κb activities. Carcinogenesis. 2011;32:1198–206.
Gasparian AV, Guryanova OA, Chebotaev DV, Shishkin AA, Yemelyanov AY, Budunova IV. Targeting transcription factor nfκb: comparative analysis of proteasome and ikk inhibitors. Cell Cycle. 2009;8:1559–66.
Palombella VJ, Rando OJ, Goldberg AL, Maniatis T. The ubiquitinproteasome pathway is required for processing the nf-κb1 precursor protein and the activation of nf-κb. Cell. 78:773–85.
Haupt Y, Maya R, Kazaz A, Oren M. Mdm2 promotes the rapid degradation of p53. Nature. 1997;387:296–9.
Gupta SC, Sundaram C, Reuter S, Aggarwal BB. Inhibiting nf-κb activation by small molecules as a therapeutic strategy. Biochim Biophys Acta (BBA) - Gene Regul Mech. 2010;1799:775–87.
Leotoing L, Chereau F, Baron S, Hube F, Valencia H, Bordereaux D, et al. A20-binding inhibitor of nuclear factor-kappab (nf-kappab)-2 (abin-2) is an activator of inhibitor of nf-kappab (ikappab) kinase alpha (ikkalpha)-mediated nf-kappab transcriptional activity. J Biol Chem. 2011;286:32277–88.
Cho W-R, Yoon H. Apple extracts attenuate tumor necrosis factor-α-induced nuclear factor-κb activation by inhibiting iκb kinase and proteasome in a549 human non-small lung carcinoma cells. Food Sci Biotechnol. 2012;21:1203–8.
White E. Life, death, and the pursuit of apoptosis. Genes Dev. 1996;10:1–15.
Singh S, Khar A. Activation of nfκb and ub-proteasome pathway during apoptosis induced by a serum factor is mediated through the upregulation of the 26 s proteasome subunits. Apoptosis. 2006;11:845–59.
Ahn J, Lee H, Kim S, Park J, Ha T. The anti-obesity effect of quercetin is mediated by the ampk and mapk signaling pathways. Biochem Biophys Res Commun. 2008;373:545–9.
Calvaruso G, Giuliano M, Portanova P, De Blasio A, Vento R, Tesoriere G. Bortezomib induces in hepg2 cells iκbα degradation mediated by caspase-8. Mol Cell Biochem. 2006;287:13–9.
Xia L, Zhang D, Du R, Pan Y, Zhao L, Sun S, et al. Mir‐15b and mir‐16 modulate multidrug resistance by targeting bcl2 in human gastric cancer cells. Int J Cancer. 2008;123:372–9.
Li Z, Zhan W, Wang Z, Zhu B, He Y, Peng J, et al. Inhibition of prl-3 gene expression in gastric cancer cell line sgc7901 via microrna suppressed reduces peritoneal metastasis. Biochem Biophys Res Commun. 2006;348:229–37.
Liang J, Pan Y, Zhang D, Guo C, Shi Y, Wang J, et al. Cellular prion protein promotes proliferation and g1/s transition of human gastric cancer cells sgc7901 and ags. FASEB J. 2007;21:2247–56.
Sun L, Wang X. Effects of allicin on both telomerase activity and apoptosis in gastric cancer sgc-7901 cells. World J Gastroenterol. 2003;9:1930–4.
Porter AG, Jänicke RU. Emerging roles of caspase-3 in apoptosis. Cell Death Differ. 1999;6.
Conflicts of interest
None
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Li, Y., Gao, H., Wang, Y. et al. Investigation the mechanism of the apoptosis induced by lactacystin in gastric cancer cells. Tumor Biol. 36, 3465–3470 (2015). https://doi.org/10.1007/s13277-014-2982-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13277-014-2982-x