Abstract
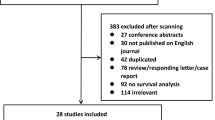
Serum Epstein-Barr virus DNA has been approved for diagnosing nasopharyngeal carcinoma (NPC). The goal of this meta-analysis was to evaluate the clinical value of the serum Epstein-Barr virus DNA in the diagnosis of NPC. The PubMed, Embase, Web of Knowledge, Chinese Wanfang Med Online, and National Knowledge Infrastructure (CNKI) databases were searched to identify suitable studies. The pooled sensitivity, specificity, positive likelihood ratio (LR+), negative likelihood ratio (LR−), and diagnostic odds ratio (DOR) of the serum Epstein-Barr virus DNA for the diagnosis of NPC were calculated. Summary receiver operating characteristic curves were used to summarize overall test performances. Meta-Disc 1.4 and Stata 12.0 softwares were used to analyze the data. A total of 2,520 patients from ten trials were subjected to meta-analysis. The summary estimates of the serum Epstein-Barr virus DNA for NPC diagnosis were as follows: sensitivity 0.69 (95 % confidence interval (CI) 0.65–0.72), specificity 0.84 (95 % CI = 0.82–0.86), LR + 4.81 (95 % CI = 2.94–7.88), LR − 0.25 (95 % CI = 0.13–0.48), DOR 24.65 (95 % CI = 12.64–48.07), and area under the summary receiver operator characteristic (SROC) curve (AUC) was 0.8979. Our study demonstrates that the serum Epstein-Barr virus DNA could be a useful tumor marker for NPC diagnosis.







Similar content being viewed by others
References
Ferlay J, Shin HR, Bray F, et al. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010;127:2893–917.
Jia WH, Huang QH, Liao J, et al. Trends in incidence and mortality of nasopharyngeal carcinoma over a 20-25 year period (1978/1983–2002) in Sihui and Cangwu counties in southern China. BMC Cancer. 2006;6:178.
Raab-Traub N, Flynn K, Pearson G, et al. The differentiated form of nasopharyngeal carcinoma contains Epstein-Barr virus DNA. Int J Cancer. 1987;39:25–9.
Whiting P, Rutjes AW, Reitsma JB, Bossuyt PM, Kleijnen J. The development of QUADAS: a tool for the quality assessment of studies of diagnostic accuracy included in systematic reviews. BMC Med Res Methodol. 2003;3:25.
Deville WL, Buntinx F, Bouter LM, et al. Conducting systematic reviews of diagnostic studies: didactic guidelines. BMC Med Res Methodol. 2002;2:9.
Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–60.
Zamora J, Abraira V, Muriel A, Khan K, Coomarasamy A. Meta-DiSc: a software for meta-analysis of test accuracy data. BMC Med Res Methodol. 2006;6:31.
Mutirangura A, Pornthanakasem W, Theamboonlers A, et al. Epstein–Barr viral DNA in serum of patients with nasopharyngeal carcinoma. Clin Cancer Res. 1998;4:665–9.
Shotelersuk K, Khorprasert C, Sakdikul S, et al. Epstein-Barr virus DNA in serum/plasma as a tumor marker for nasopharyngeal cancer. Clin Cancer Res. 2000;6:1046–51.
Chan KH, Gu YL, Ng F, et al. EBV specific antibody-based and DNA-based assays in serologic diagnosis of nasopharyngeal carcinoma. Int J Cancer. 2003;105:706–9.
Fan H, Nicholls J, Chua D, et al. Laboratory markers of tumor burden in nasopharyngeal carcinoma: a comparison of viral load and serologic tests for Epstein-Barr virus. Int J Cancer. 2004;112:1036–41.
Krishna SM, James S, Kattoor J, et al. Serum EBV DNA as a biomarker in primary nasopharyngeal carcinoma of Indian origin. Jpn J Clin Oncol. 2004;34:307–11.
Luo YL, Ou GP, Chi PD, et al. Combined determination of Epstein-Barr virus-related antibodies and antigens for diagnosis of nasopharyngeal carcinoma. Chin J China. 2009;28:96–9.
Mo WN, Tang AZ, Zhou L, et al. Analysis of Epstein-Barr viral DNA load, EBV-LMP2 specific cytotoxic T-lymphocytes and levels of CD4+/CD25+ T cells in patients with nasopharyngeal carcinomas positive for IgA antibody to EBV viral capsid antigen. Chin Med J (Engl). 2009;122:1173–8.
Tan YJ, Su XK, Cui JH. Comparison of detection of serum VCA-lgA, EA-IgA and EBV-DNA in nasopharyngeal carcinoma patient. Chongqing Med J. 2010;39:703–6.
Wei K, Xu Y, Liu J, Zhang W, Liang Z. No incidence trends and no change in pathological proportions of nasopharyngeal carcinoma in Zhongshan in 1970-2007. Asian Pac J Cancer Prev. 2010;11:1595–9.
Zhang LW, Luo BQ, Dou XQ, et al. Quantitative analysis of Epstein-Barr virus DNA in saliva, blood serum and peripheral blood cells in patients with nasopharyngeal carcinoma. Chin J Otorhinolaryngol Skull Base Surg. 2012;18:24–7.
Rosenfeld RM, Shiffman RN. Clinical practice guidelines: a manual for developing evidence-based guidelines to facilitate performance measurement and quality improvement. Otolaryngol Head Neck Surg. 2006;135:S1–28.
Conflicts of interest
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sun, D., Yang, Z., Fu, Y. et al. Clinical value of serum Epstein-Barr virus DNA assay in the diagnosis of nasopharyngeal carcinoma. Tumor Biol. 35, 8787–8793 (2014). https://doi.org/10.1007/s13277-014-2148-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13277-014-2148-x