Abstract
Background
Emerging evidence suggests that rhaponticin, a stilbene monomeric compound isolated from North China rhubarb, has been shown to exhibit significant biological activity against tumors. However, the anticancer effects and mechanisms of rhaponticin in tongue squamous cell carcinoma (TSCC) remain elusive.
Objective
We investigated the changes of migration and invasion abilities and EMT progression of TSCC cells treated with different concentrations of rhaponticin under hypoxia, as well as the possible mechanisms, in order to initially explore the effects of rhaponticin on the biological characteristics of TSCC cells under hypoxia.
Results
The number of cell migration and invasion was prominently increased, E-cadherin protein was down-regulated, and N-cadherin and HIF-1α protein expression was elevated under hypoxia. Rhaponticin intervention strikingly prevented the increased abilities of migration and invasion and EMT of TSCC cells under hypoxia. This was followed by further validation finding that rhaponticin indeed leads to reduced HIF-1α post-transcriptional activity. Mechanistically, rhaponticin may bind to aryl-hydrocarbon nuclear translocator (ARNT) domain of HIF-1α.
Conclusions
Rhaponticin repressed the invasion and migration abilities and EMT process of TSCC cells under a hypoxic environment in vitro by targeted suppression of HIF-1α.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Tongue squamous cell carcinoma (TSCC) is one of the most common head and neck malignant tumors in the clinic, with more than 350,000 patients developing TSCC every year worldwide, which is mainly considered to be associated with smoking, alcohol consumption, physical and mechanical stimuli, viral infection, and other factors, with high recurrence and metastasis rates (Ohta and Yoshimura 2019; Lau et al. 2021). In recent decades, the 5-year survival rate of squamous cell carcinoma of the tongue is still less than 50%, although with the continuous improvement of surgery and combined treatments such as radiotherapy and chemotherapy (Nóbrega et al. 2018). Besides, the remarkable biological propensity for local invasion and the high incidence of early cervical lymph node metastasis (~ 40%) in TSCC are important factors contributing to the poor prognosis of patients with TSCC (Rani et al. 2020; Sano and Myers 2007). Although many studies have been published on the mechanism of invasion and metastasis of TSCC, the factors and specific mechanisms that effectively repress the invasion and metastasis of TSCC remain unclear. Therefore, an in-depth study of the mechanism of invasion and metastasis of TSCC, a search for therapeutic targets in tumors, and the development of effective therapeutic drugs are of great significance to improve the prognosis of patients with TSCC.
Cancer metastasis begins with the loss of tumor cell adhesion, and tumor cells migrate out of the primary foci and invade surrounding tissues (Fares et al. 2020). In recent years, numerous studies have shown that epithelial mesenchymal transitions (EMT) play an important role in the progression of a variety of tumors, and epithelial cancers achieve invasion and metastasis through EMT (Campbell 2018). During EMT, epithelial cells lose their epithelial phenotypes such as cell polarity and loss of connection to the basement membrane, and transform into a long spindle shaped cell low proliferation state that increases with cell migration, invasion, and survival (Kang et al. 2019; Pastushenko and Blanpain 2019). Concomitant with changes in cellular phenotype, there are changes in cellular markers. Epithelial markers such as E-cadherin and keratins showed decreased expression, and interstitial markers such as vimentin, fibronectin and N-cadherin showed increased expression (Wong et al. 2018; Pal et al. 2018). The microenvironment of solid tumors has a hypoxic state due to the inability of the oxygen supply of tumor cells to meet their oxygen consumption, resulting in insufficient oxygen and nutrient supply to tumor cells (Jing et al. 2019). Hypoxia is closely related to the metastasis of cancer due to its ability to stimulate angiogenesis and lymph-angiogenesis under hypoxia, which allows cancer cells to escape from the unfavorable tumor microenvironment, spread to secondary sites (Ma et al. 2021). It has been confirmed that hypoxic microenvironment could drive some oncogenes overexpression and then promote cancer cell development, in which hypoxia-inducible factor-1α (HIF-1α) plays an indispensable role (Tirpe et al. 2019). As the initiator and common pathway of body self-protection, HIF-1α is involved in the regulation processes of hypoxic stress, angiogenesis, and cell behaviors by activating the expression of a variety of stress proteins in response to hypoxia (Semenza 2007; Merelli et al. 2021). Several studies have confirmed that aberrantly high HIF-1α expression is involved in the regulation of cell invasion, metastasis, and other malignancies, such as hepatocellular carcinoma (Li et al. 2021) and oral cancer (Qian et al. 2019). Further, Liang et al. (Liang et al. 2011) demonstrated that the high expression level of HIF-1α was closely related to the stage, efficacy, and metastasis of TSCC. Zhou et al. (Zhou et al. 2015) discovered that downregulating HIF-1α expression in TSCC cells could induce apoptosis and inhibit cell growth and invasion. These data suggest that HIF-1α may serve as a target to improve the therapeutic efficacy of TSCC.
Stilbenoids are one of the major active components of commonly used traditional Chinese medicines (TCM), such as Polygonum multiflorum, rhubarb, and Polygonum cuspidatum, which have many biological activities, such as antibacterial, anti-inflammatory, antitumor, antioxidant, hypoglycemic, and hypolipidemic properties (Akinwumi et al. 2018). Rhaponticin, a stilbene monomeric compound isolated from North Chinese rhubarb, has hypoglycemic, antithrombotic, anti-allergic, antitumor, and other effects (Chen et al. 2020). Suresh et al. (Mickymaray et al. 2021) suggested that rhaponticin showed effective cytotoxicity and basically inhibited the growth of osteosarcoma cells. Wang et al. (Wang et al. 2021b) confirmed that oral administration of rhaponticin in mice prevented the occurrence of lung cancer and improved the histopathological changes of lung tissue. In addition, a recent study suggested that rhaponticin exhibits potent anti-metastatic and anti-angiogenic activities by inhibiting the HIF-1α signaling pathway (Kim and Ma 2018). Nevertheless, whether rhaponticin is involved in regulating TSCC development and the underlying mechanisms remain incompletely explored.
Here, we investigated the changes of migration and invasion abilities and EMT progression of TSCC cells treated with different concentrations of rhaponticin under hypoxia, as well as the possible mechanisms, in order to initially explore the effects of rhaponticin on the biological characteristics of TSCC cells under hypoxia.
Materials and methods
Cell culture and grouping
The human TSCC cell lines CAL-27 and SCC-9, and normal human oral keratinocyte (NHOK) were all purchased from Shanghai Institute of cell technology, Chinese Academy of Sciences (Shanghai, China). TSCC cells were seeded in 50 mL culture flasks containing 10% fetal bovine serum, l × 105 U/L penicillin, and 100 mg/L streptomycin in DMEM. Cell culture flasks were placed in a cell culture incubator at 37 °C with 5% CO2 saturated humidity, and logarithmic growth phase cells were taken for experiments. To simulate the low oxygen microenvironment inside the tumor, the incubator was adjusted to a saturated humidity of 37 °C, 5% CO2, 94% N2, and 1% O2 for hypoxic condition culture. Then, CAL-27 cells were randomly divided into 4 groups, including 0 µM rhaponticin, 5 µM rhaponticin, 10 µM rhaponticin, and 50 µM rhaponticin. For the 0 µM group, CAL-27 was cultured normally in hypoxia. For the 5 µM group, a final concentration of 5 µM of rhaponticin (Macklin Biochemical Co., Ltd., Shanghai, China) was added to the cells for incubation for 48 h under hypoxia. For the 10 µM group, a final concentration of 10 µM of rhaponticin was added to the cells for incubation for 48 h under hypoxia. For the 50 µM group, a final concentration of 50 µM of rhaponticin was added to the cells for incubation for 48 h under hypoxia. Cells were collected for further analysis.
Cell transfection
Small interfering RNA for HIF-1α was purchased from Shanghai Jima Pharmaceutical Technology Co., Ltd. When cells were 70% confluent, si-HIF-1α was transfected into CAL-27 cells using lipofectamineTM3000 transfection reagent according to the manufacturer’s instructions. Subsequently, the transfection efficiency of si-HIF-1α was evaluated using RT-qPCR and Western blotting, respectively. Stably transfected cells were harvested 48 h after transfection for subsequent experiments. Subsequently, the transfected CAL-27 cells were divided into four groups, including si-NC, si-NC + rhaponticin, si- HIF-1α, si-HIF-1α + rhaponticin. For the si-NC group, CAL-27 cells were transfected with si-NC for 48 h. For the si-NC + rhaponticin group, 50 μM of rhaponticin treatment was given after transfection of si-NC for 48 h under hypoxia. For the si-HIF-1α group, CAL-27 cells were transfected with si-HIF-1α for 48 h. For the si-HIF-1α + rhaponticin group, 50 μM of rhaponticin treatment was given after transfection of si-HIF-1α for 48 h under hypoxia. Cells were collected for further analysis.
Cell viability assay
CAL-27 cells and NHOK were treated under hypoxia with different concentrations of rhaponticin (0, 5, 10, 50, 100, 200, and 500 μM), and then evaluated cell activity 24 h later. The viability of cells in each group was assessed according to the CCK-8 cell proliferation activity assay kit instructions. The OD value at 450 nm was measured using a microplate reader (bio rad, USA).
Cell invasion assay
Matrigel gel at 1 mg/mL was pre-plated in Transwell chambers. CAL-27 or SCC-9 cells from each group (5.0 × 105/mL) were seeded in the upper chamber, and 500 μL of DMEM medium (containing 10% fetal bovine serum) was added to the lower chamber. After routine culture until Matrigel was completely degraded, cells were fixed with 0.25% glutaraldehyde for 20 min and stained with 0.1% crystal violet for 30 min. Five fields were randomly selected, and the number of penetrating cells/fields was calculated. Experiments were repeated 3 times and averaged.
Cell migration assay
Cells were streaked in the central area of cell growth with a sterile pipette tip and then rinsed with PBS to remove dead cells. The scratch width was observed under a microscope at 24 h after scratching and was photographed and recorded.
RT-qPCR
Cells from the control, si-NC, and si-HIF-1α groups were collected, the RNA concentration of each sample was determined, and the RNA was reverse transcribed into cDNA following the reverse transcription kit protocol. This cDNA was then used as a template for HIF-1α mRNA amplification on Bio-Rad CFX96 Real time PCR (Bio-rad Company, USA). RT-PCR reaction conditions are: pre-denaturation at 95 °C for 30 s, 39 cycles of denaturation at 95 °C for 5 s, annealing at 60 °C for 5 s, and extension at 65 °C for 5 s. The primers are listed in Table 1. GAPDH was used as an internal reference and relative expression levels were calculated using the 2−ΔΔCT method.
Western blotting
Each group of cells with different treatments was collected, and total protein was extracted by adding Ripa lysis solution containing protease inhibitors. Protein concentrations were determined using the BCA protein assay kit (Thermofisher, USA) and subsequently electrophoresed using 8–12% SDS polyacrylamide gels. A semi-dry method was employed to transfer proteins to nitrocellulose membranes, and then blocking was subsequently performed using 1% bovine serum albumin (BSA). Primary antibodies were diluted by PBST to working solution concentrations and incubated with proteins overnight. Secondary antibodies were diluted by PBST to working solution concentration after incubating proteins for 1 h in a constant temperature shaker protected from light at 37 °C. The gray values of protein bands were finally scanned with ImageJ setup software. The information of antibodies is listed in Table 2.
Molecular docking assay
The three-dimensional structure of HIF-1α (PDB code: 4ZPR) was obtained from the Protein Data Bank (http://www.wwpdb.org/). The 3D structure of rhaponticin was downloaded from PubChem chemical library. The potential binding 3D model of rhaponticin to HIF-1α was subsequently predicted using Schrodinger suite 2021 analysis.
Statistical analysis
SPSS 22.0 and GraphPad prism 8.0 were used for data analysis and mapping. All the data were described as the mean ± SEM. Student's t-test was used for the comparison of two groups, and analysis of variance was used for the comparison of multiple groups, with P < 0.05 considered significant.
Results
HIF-1α mediates the invasive phenotype of TSCC cells in a hypoxic environment
We subjected TSCC cell lines CAL-27 and SCC-9 cells to normoxic (21% O2) and hypoxic (1% O2) conditions for 24 h, respectively. Subsequently, cell scratch and Transwell assays were applied to check the migration and invasion abilities of CAL-27 and SCC-9 cells in vitro under hypoxia. Figure 1A results displayed that at the beginning of the experiment, the scratch width was basically consistent in the normoxia and hypoxia groups. After 24 h of hypoxic culture, it could be observed that the ability of CAL-27 and SCC-9 cells to move towards the blank of the intermediate scratch was preeminently increased, indicating that the migration of TSCC cells was encouraged by hypoxia. Further, the results of Transwell assay indicated that the number of CAL-27 and SCC-9 cells that penetrated polycarbonate membranes was conspicuously lower in cultured under hypoxic conditions for 24 h than in normoxia, suggesting that hypoxic environments can facilitate the invasive capacity of human TSCC cells in vitro (Fig. 1B). Next, to explore whether the effect of hypoxic environment on invasion and migration of TSCC cells was associated with EMT alterations, we employed Western blotting to check the expression levels of EMT-related proteins. As can be observed from Fig. 1C, E-cadherin protein expression was materially decreased and N-cadherin expression was conspicuously upregulated in CAL-27 and SCC-9 cells under hypoxia compared with normoxia. It was illustrated that culturing TSCC cells under hypoxia was able to accelerate EMT progression. In addition, as HIF-1α protein expression was strikingly upregulated in TSCC cells following hypoxia induction (Fig. 1C), we hypothesized that increased HIF-1α may be a potential mechanism by which hypoxia accelerates TSCC cell migration, invasion, and EMT.
HIF-1α mediates the invasive phenotype of TSCC cells in a hypoxic environment. A Cell scratch assay assessment of the migration ability of TSCC cells under normoxia (21% O2) and hypoxia (1% O2). B Transwell assay assessment of the invasion ability of TSCC cells under normoxia (21% O2) and hypoxia (1% O2). C Western blotting assessment of the protein levels of HIF-1α, E-cadherin, and N-cadherin in TSCC cells under normoxia (21% O2) and hypoxia (1% O2). n = 3. Data are presented as the means ± SEM. p value was based on Student’s t-test. **P < 0.01, ***P < 0.001 vs. Normoxia group
Rhaponticin suppresses the invasive phenotype of TSCC in a hypoxic environment
Subsequently, to explore the effect of rhaponticin on TSCC cell invasion and migration, CAL-27 cells were treated under hypoxia with different concentrations of rhaponticin (0, 5, 10, 25, 50, 100, 200 μM), and then evaluated cell activity 24 h later. Figure 2A displays the chemical structural formula of rhaponticin. CCK8 results revealed that after different concentrations of rhaponticin intervened on CAL-27 cells, the cell viability was gradually decreased in a dose-dependent manner. Moreover, the IC50 value of rhaponticin against CAL-27 cells was 82.70 μM (Fig. 2B). These data suggested that rhaponticin suppressed CAL-27 cell viability but had no significant effect on NHOK when the concentration lower than 50 μM. Therefore, in order to exclude the influence of rhaponticin on the cell migration test results by inhibiting cell proliferation, rhaponticin at 0, 5, 10, and 50 μM was selected to do subsequent studies. Meanwhile, the cytotoxicity of rhaponticin in normal oral cells (NHOK) was also assessed; the results showed that IC50 value of rhaponticin against NHOK was 241.0 μM (Fig. 2B). Subsequently, the results of cell scratch assay suggested that after the treatment of CAL-27 cells with 0, 5, 10, and 50 μM of rhaponticin, the cell migration ability would be significantly inhibited with increasing concentrations of rhaponticin (Fig. 2C). Similarly, Transwell results displayed that rhaponticin dose-dependently decreased the invasive cell number of CAL-27 cells under hypoxia (Fig. 2D). Further, we also found that rhaponticin upregulated E-cadherin protein levels and repressed N-cadherin protein levels in CAL-27 cells at a hypoxic environment in a concentration-dependent manner (Fig. 2E). In short, it is indicated that rhaponticin could observably prevent the increase of migration and invasion abilities and EMT of CAL-27 cells under a hypoxic environment.
Rhaponticin suppresses the invasive phenotype of TSCC in a hypoxic environment. A Chemical structure formula of rhaponticin. B CCK-8 assay assessment of the viability of CAL-27 cells and NHOK intervened with rhaponticin (0, 5, 10, 50, 100, 200, and 500 μM) under hypoxia (1% O2) for 24 h. C Cell scratch assay assessment of the migration ability of CAL-27 cells intervened with rhaponticin (0, 5, 10, 50 μM) under hypoxia (1% O2) for 24 h. D Transwell assay assessment of the invasion ability of CAL-27 cells intervened with rhaponticin (0, 5, 10, 50 μM) under hypoxia (1% O2) for 24 h. E Western blotting assessment of the E-cadherin and N-cadherin protein levels in CAL-27 cells intervened with rhaponticin (0, 5, 10, 50 μM) under hypoxia (1% O2) for 24 h. n = 3. Data are presented as the means ± SEM. p value was based on one-way analysis of variance. *P < 0.05, **P < 0.01, ***P < 0.001 vs. 0 μM group
Knockdown of HIF-1α counteracts the effect of rhaponticin on TSCC in a hypoxic environment
Next, to explore whether the inhibitory effect of rhaponticin on the aggressive phenotype of TSCC was associated with HIF-1α, we stably knocked-down HIF-1α expression in CAL-27 cells and then examined whether HIF-1α knockdown has an impact on rhaponticin’s therapeutic effect. RT-qPCR and Western blotting were applied to examine the knockdown efficiency of si-HIF-1α, and the results suggested that the mRNA and protein levels of HIF-1α in CAL-27 cells were reduced about threefold after si-HIF-1α transfection (Fig. 3A and B). Moreover, cell scratch and Transwell results suggested that the knockdown of HIF-1α decreased the number of migrated and invaded cells under hypoxia. However, the knockdown of HIF-1α based on the administration of rhaponticin treatment had less significant changes in cell migration and invasion abilities than that of untreated rhaponticin (Fig. 3C and D). Besides, HIF-1α knockdown remarkably raised the protein expression of E-cadherin and suppressed the protein level of N-cadherin, whereas there were no significant changes in the protein levels of E-cadherin and N-cadherin in CAL-27 cells treated with rhaponticin based on HIF-1α knockdown (Fig. 3E). In accordance with this, we found that rhaponticin intervention and HIF-1α knockdown had the same effect on the invasive phenotype of CAL-27 cells, whereas HIF-1α knockdown counteracted the promoting effect of rhaponticin intervention on the invasive behavior of CAL-27, speculating that rhaponticin may play a regulatory role using the inhibition of as a mechanism. Furthermore, consistent results were also shown in the SCC-15 cell line (Supplementary Figure S1), which corroborated the finding that the suppressive effect of rhaponticin on invasive phenotype of TSCC in a hypoxic environment relies on the inhibition of HIF-1α.
Knockdown of HIF-1α counteracts the effect of rhaponticin on CAL-27 cells in a hypoxic environment. A RT-qPCR assessment of the mRNA level of HIF-1α in CAL-27 cells transfected with si-NC or si-HIF-1α under hypoxia (1% O2) for 24 h. B Western blotting assessment of the protein level of HIF-1α in CAL-27 cells transfected with si-NC or si-HIF-1α under hypoxia (1% O2) for 24 h. C Cell scratch assay assessment of the migration ability of CAL-27 cells intervened with rhaponticin (10 μM) or/and transfected with si-HIF-1α under hypoxia (1% O2) for 24 h. D Transwell assay assessment of the invasion ability of CAL-27 cells intervened with rhaponticin or/and transfected with si-HIF-1α under hypoxia (1% O2) for 24 h. E: Western blotting assessment of the E-cadherin and N-cadherin protein levels in CAL-27 cells intervened with rhaponticin (10 μM) or/and transfected with si-HIF-1α under hypoxia (1% O2) for 24 h. n = 3. Data are presented as the means ± SEM. p value was based on one-way analysis of variance. ***P < 0.001 vs. si-NC group
Rhaponticin inhibits HIF-1α transcriptional activity by binding to the ARNT domain of HIF-1α
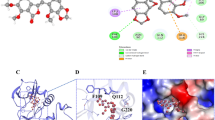
Subsequently, we aimed to further confirm whether rhaponticin inhibits HIF-1α expression by binding to its functional domain. Different doses of rhaponticin were first intervened on CAL-27 cells for 24 h, and then RT-qPCR and Western blotting were employed to measure the mRNA and protein levels of HIF-1α in CAL-27 cells, respectively. Figure 4A results suggested that there was no significant difference in the mRNA level changes of HIF-1α after rhaponticin intervention in both CAL-27 and SCC-15 cells. However, the results in Fig. 4B illustrated that rhaponticin intervention down-regulated the protein level of HIF-1α in a dose-dependent manner, suggesting that rhaponticin may inhibit HIF-1α expression at the post-transcriptional level. It has been reported that HIF-1α can regulate the levels of hypoxia-response element (HRE) that drive specific genes. Therefore, the present study next evaluated the reporter expression of HIF-1α-HRE with a dual luciferase reporter assay. Further findings showed that rhaponticin indeed led to a decrease in HIF-1α transcriptional activity by transfecting CAL-27 and SCC-15 cells with a HIF-1α-HRE-luciferase reporter construct (Fig. 4C). Studies have confirmed that the aryl-hydrocarbon nuclear translocator (ARNT) domain is required for HIF-1α subunits to form heterodimers. We found that rhaponticin might bind to the ALA95 and ASP97 protein sites in the ARNT structure by Autodock tool prediction. Figure 4D is shown as a molecular docking diagram of rhaponticin with HIF-1α, further confirming that the binding site of rhaponticin with HIF-1α is well characterized. The above studies confirmed that rhaponticin was able to inhibit the post-transcriptional expression level of HIF-1α by binding at the ARNT domain of HIF-1α.
Rhaponticin inhibits HIF-1α transcriptional activity by binding to the ARNT domain of HIF-1α. A RT-qPCR assessment of the mRNA levels of HIF-1α in CAL-27 and SCC-15 cells intervened with rhaponticin (0, 5, 10, 50 μM) under normoxia (21% O2) for 24 h. B Western blotting assessment of the HIF-1α protein level in CAL-27 and SCC-15 cells intervened with rhaponticin (0, 5, 10, 50 μM) under normoxia (21% O2) for 24 h. C Luciferase reporter experiments detection of transcriptional activity of HIF-1α-HRE-luciferase reporter construct in CAL-27 and SCC-15 cells intervened with rhaponticin (0, 5, 10, 50 μM) under normoxia (21% O2) for 24 h. D Molecular docking diagram of rhaponticin with HIF-1α. n = 3. Data are presented as the means ± SEM. p value was based on one-way analysis of variance. *P < 0.05, ***P < 0.001 vs. 0 μM group
Discussion
TSCC, as a tumor with an extremely high incidence in the oral and maxillofacial region, has a 5-year survival rate of less than 50%, although with the continuous improvement of surgery as well as comprehensive treatments such as radiotherapy and chemotherapy (Carr et al. 2021). Among them, the significant biological predisposition to local invasion and the high incidence of early cervical lymph node metastasis are important factors contributing to the poor prognosis of patients with TSCC (Wang et al. 2021a). In this study, we found that the invasion and migration abilities as well as EMT of TSCC cells were significantly enhanced under hypoxia. During tumor development, the oxygen supply of tumor cells cannot meet its oxygen consumption, resulting in insufficient oxygen and nutrient supply to tumor cells, forming a tumor hypoxic microenvironment (Boutilier and Elsawa 2021; Liu et al. 2020). The tumor hypoxic microenvironment was confirmed to play an important role during tumor progression and metastasis. Hypoxic tumor cells affect tumor progression and metastasis by altering oncogene expression, genomic instability, tumor angiogenesis, as well as EMT (Sugita et al. 2021). In which EMT is a rapid and reversible change in cell phenotype that refers to the detachment of the link between the epithelial cells and the basement membrane and the transformation to mesenchymal cells after they have been stimulated by specific factors such as extracellular factors (Cho et al. 2019). The current studies have found that EMT is the ‘first step’ in tumor metastasis, and tumor cells can acquire higher invasion ability and metastasis ability after EMT occurs. Loss or downregulation of E-cadherin expression is considered to be a key important step in the process of tumorigenesis and development as well as EMT (Shash et al. 2021). In addition, one of the EMT mechanisms is the conversion between cadherins, and the main conversion process is manifested as the conversion of E-cadherin into N-cadherin (Kaszak et al. 2020). E-cadherin suppresses tumorigenesis, invasion as well as metastasis, while N-cadherin promotes tumorigenesis, invasion as well as metastasis (Na et al. 2020). Loss or downregulation of E-cadherin expression, directly and indirectly, affects N-cadherin expression. Zhang et al. (Zhang et al. 2021) demonstrated that hypoxia-induced EMT plays a key role in the ability of cervical cancer to invade surrounding tissues. Takeshi et al. (Kaneko et al. 2016) revealed evidence that under hypoxia oral squamous cell carcinoma cell lines exhibit EMT being promoted. Further, Zhong et al. (Zhong et al. 2018) suggested that the hypoxic microenvironment plays an important role in the metastatic process of TSCC tumors, as indicated by the significant induction of EMT in TSCC cells. Similarly, our study discovered that TSCC cell migration and invasion were obviously enhanced, E-cadherin protein expression was inhibited, and N-cadherin protein levels were upregulated under a hypoxic environment.
There are obvious hypoxic regions in most solid tumors, while tumor cells can still continuously proliferate and undergo invasion and metastasis under a hypoxic microenvironment, in which HIF-1α plays a pivotal role (Rashid et al. 2021). HIF-1α is a key factor in the adaptive regulation of cells in hypoxic environments and is important in regulating tumor cell metabolism, angiogenesis, proliferation, invasion, and chemoradiotherapy resistance (Albadari et al. 2019). At present, abnormally high expression of HIF-1α has been found in various malignancies, such as breast cancer (Chen et al. 2019), renal cancer (Cowman et al. 2020), and esophageal squamous cell carcinoma (Hu et al. 2020), and its level can significantly affect the prognostic efficacy. Tetsuya et al. (Hirabayashi et al. 2017) suggested that HIF-1α is closely related to TSCC progression and is associated with TNM stage, and lymph node metastasis. Amanda et al. (Siu et al. 2013) found that after HIF-1α was activated in TSCC, it could induce cell growth and angiogenesis and promote the epithelial mesenchymal transition process. In this study, we found that when TSCC cells were cultured with hypoxia, HIF-1α expression was particularly elevated, and knockdown of HIF-1α could prevent the aggressive phenotype of TSCC cells, implying its potential as a molecular target for lung cancer treatment.
TCM is a specific traditional system in China, with the penetration and influence of modern science and technology, especially the development of natural product chemistry, many of the chemical components of TCM have been elucidated, and the TCM examination and evaluation also by the content of morphology and traditional pharmacognosy has gradually increased the methods and means of chemical analysis (Zhou 2010). In recent years, an increasing number of studies have set out to find new antitumor treatment options from pure natural phytocompounds. Rhaponticin is a stilbenoid isolated from Polygonum aviculare L. and has been confirmed by pharmacological tests to have a tumor-suppressive effect. Li et al. (Li et al. 2014) showed that rhaponticin inhibited intracellular fatty acid synthase activity and subsequently induced apoptosis in human breast cancer MCF-7 cells. This study confirmed that rhaponticin could effectively inhibit TSCC cell migration, invasion, and EMT progression, and the inhibitory effect was also enhanced with the increase of the effect concentration. In addition, 50 μM of rhaponticin caused an approximate 30% reduction in TSCC cell viability but more than 50% reduction in TSCC cell migration and invasion, which suggested that the inhibition of TSCC cell aggressiveness by rhaponticin is not dependent on its inhibitory effect on cell proliferation. Kim et al. (Kim and Ma 2019) demonstrated that Rhubarb treatment reduced the production of proangiogenic factors in human fibrosarcoma cells under normoxic and hypoxic conditions and inhibited the hypoxia-inducible activation of the HIF-1α pathway, of which rhaponticin is one of the major active components. Moreover, an experimental result suggested that rhaponticin inhibited the metastatic potential of malignant cancer cells, including breast cancer cells MDA-MB231 and fibrosarcoma cells HT1080, by inhibiting the HIF-1α pathway and regulating EMT-related proteins (Kim and Ma 2018). The above studies suggest that the regulation of rhaponticin involved in tumor development may be related to the inhibition of HIF-1α activation. The results of the present study found that rhaponticin intervention and HIF-1α knockdown had the same effect on the invasive phenotype of TSCC cells, whereas si-HIF-1α and rhaponticin co-treatment did not significantly change the invasive phenotype compared with si-HIF-1α alone transfected cells. From this, we speculate that rhaponticin may regulate the aggressive phenotype of TSCC cells by inhibiting HIF-1α expression as one of the mechanisms. However, when HIF-1α has already been knocked-down results in a lack of targets for rhaponticin. This was followed by further validation finding that rhaponticin indeed leads to decreased HIF-1α transcriptional activity. In addition, rhaponticin was found to potentially bind to ALA95 and ASP97 protein sites in the ARNT domain of HIF-1α by Autodock tool prediction. Previous studies have established that the activity of HIF-1 requires binding to the ARNT domain to be achieved, and interfering with or downregulating endogenous ARNT proteins decreases HIF-1 transcriptional activity (Isaacs et al. 2004). Our results provide proof of the principle that rhaponticin is a naturally effective drug for the development of TSCC therapy by targeting HIF-1α.
Nevertheless, the present study has several limitations. Whether rhaponticin affects other biological properties of TSCC cells, such as cell growth arrest or apoptosis, remains to be determined. Whether rhaponticin plays a role in radio-sensitization, and animal experiments are missing. In the following experiments we intended to use CAL-27 cells to construct a tumor bearing mouse model to further deeply explore the regulation of rhaponticin as well as the underlying mechanism, which would be the next issue to be addressed in this study.
In conclusion, the present study demonstrated that rhaponticin repressed the invasive and migration ability and EMT process of TSCC cells under a hypoxic environment in vitro. Mechanistically, rhaponticin could target the ARNT domain that binds HIF-1α, which in turn inhibits HIF-1α expression and further affects the invasive properties of TSCC cells under a hypoxic environment. This will likely provide an experimental basis for the application of rhaponticin to the treatment of clinical tumors.
Data availability
The data used to support the findings of this study are available from the corresponding author upon request.
References
Akinwumi BC, Bordun KM, Anderson HD (2018) Biological Activities of Stilbenoids. Int J Mol Sci 19(3):792
Albadari N, Deng S, Li W (2019) The transcriptional factors HIF-1 and HIF-2 and their novel inhibitors in cancer therapy. Expert Opin Drug Discov 14:667–682
Boutilier AJ, Elsawa SF (2021) Macrophage polarization states in the tumor microenvironment. Int J Mol Sci 22:6995
Campbell K (2018) Contribution of epithelial-mesenchymal transitions to organogenesis and cancer metastasis. Curr Opin Cell Biol 55:30–35
Carr BR, Gulko JA, Neal TW, Schlieve T (2021) Commentary: squamous cell carcinoma of the tongue in young patients: a case series and literature review. J Oral Maxillofac Surg 79:2378–2379
Chen F et al (2019) Extracellular vesicle-packaged HIF-1α-stabilizing lncRNA from tumour-associated macrophages regulates aerobic glycolysis of breast cancer cells. Nat Cell Biol 21:498–510
Chen D et al (2020) Metabolism of rhaponticin and activities of its metabolite, rhapontigenin: a review. Curr Med Chem 27:3168–3186
Cho ES, Kang HE, Kim NH, Yook JI (2019) Therapeutic implications of cancer epithelial-mesenchymal transition (EMT). Arch Pharm Res 42:14–24
Cowman SJ et al (2020) Macrophage HIF-1α Is an independent prognostic indicator in kidney cancer. Clin Cancer Res 26:4970–4982
Fares J et al (2020) Molecular principles of metastasis: a hallmark of cancer revisited. Signal Transduct Target Ther 5:28
Hirabayashi T, Takahashi H, Watanabe M, Tachibana T (2017) Establishment and characterization of a squamous cell carcinoma cell line, designated hZK-1, derived from a metastatic lymph node tumor of the tongue. Hum Cell 30:319–326
Hu X et al (2020) HIF-1α promotes the metastasis of esophageal squamous cell carcinoma by targeting SP1. J Cancer 11:229–240
Isaacs JS, Jung YJ, Neckers L (2004) Aryl hydrocarbon nuclear translocator (ARNT) promotes oxygen-independent stabilization of hypoxia-inducible factor-1alpha by modulating an Hsp90-dependent regulatory pathway. J Biol Chem 279:16128–16135
Jing X et al (2019) Role of hypoxia in cancer therapy by regulating the tumor microenvironment. Mol Cancer 18:157
Kaneko T et al (2016) Hypoxia-induced epithelial-mesenchymal transition is regulated by phosphorylation of GSK3-β via PI3 K/Akt signaling in oral squamous cell carcinoma. Oral Surg Oral Med Oral Pathol Oral Radiol 122:719–730
Kang X, Wang J, Li C (2019) Exposing the underlying relationship of cancer metastasis to metabolism and epithelial-mesenchymal transitions. iScience 21:754–772
Kaszak I et al (2020) Role of cadherins in cancer-a review. Int J Mol Sci 21:7624
Kim A, Ma JY (2018) Rhaponticin decreases the metastatic and angiogenic abilities of cancer cells via suppression of the HIF-1α pathway. Int J Oncol 53:1160–1170
Kim A, Ma JY (2019) Piceatannol-3-O-β-D-glucopyranoside (PG) exhibits in vitro anti-metastatic and anti-angiogenic activities in HT1080 malignant fibrosarcoma cells. Phytomedicine 57:95–104
Lau L et al (2021) Histopathologic prognostic indices in tongue squamous cell carcinoma. Eur Arch Otorhinolaryngol 278:2461–2471
Li P, Tian W, Wang X, Ma X (2014) Inhibitory effect of desoxyrhaponticin and rhaponticin, two natural stilbene glycosides from the Tibetan nutritional food Rheum tanguticum Maxim. ex Balf., on fatty acid synthase and human breast cancer cells. Food Funct 5:251–256
Li Q et al (2021) HIF-1α-induced expression of m6A reader YTHDF1 drives hypoxia-induced autophagy and malignancy of hepatocellular carcinoma by promoting ATG2A and ATG14 translation. Signal Transduct Target Ther 6:76
Liang X et al (2011) Hypoxia-inducible factor-1 alpha, in association with TWIST2 and SNIP1, is a critical prognostic factor in patients with tongue squamous cell carcinoma. Oral Oncol 47:92–97
Liu Y et al (2020) Development and validation of a hypoxia-immune-based microenvironment gene signature for risk stratification in gastric cancer. J Transl Med 18:201
Ma Z et al (2021) Targeting hypoxia-inducible factor-1-mediated metastasis for cancer therapy. Antioxid Redox Signal 34:1484–1497
Merelli A, Repetto M, Lazarowski A, Auzmendi J (2021) Hypoxia, oxidative stress, and inflammation: three faces of neurodegenerative diseases. J Alzheimers Dis 82:S109-s126
Mickymaray S et al (2021) Rhaponticin suppresses osteosarcoma through the inhibition of PI3K-Akt-mTOR pathway. Saudi J Biol Sci 28:3641–3649
Na TY, Schecterson L, Mendonsa AM, Gumbiner BM (2020) The functional activity of E-cadherin controls tumor cell metastasis at multiple steps. Proc Natl Acad Sci U S A 117:5931–5937
Nóbrega TD et al (2018) Clinicopathological evaluation and survival of patients with squamous cell carcinoma of the tongue. Med Oral Patol Oral Cir Bucal 23:e579–e587
Ohta K, Yoshimura H (2019) Squamous cell carcinoma of the dorsal tongue. CMAJ 191:E1310
Pal M, Bhattacharya S, Kalyan G, Hazra S (2018) Cadherin profiling for therapeutic interventions in epithelial mesenchymal transition (EMT) and tumorigenesis. Exp Cell Res 368:137–146
Pastushenko I, Blanpain C (2019) EMT transition states during tumor progression and metastasis. Trends Cell Biol 29:212–226
Qian C, Dai Y, Xu X, Jiang Y (2019) HIF-1α regulates proliferation and invasion of oral cancer cells through Kv3.4 channel. Ann Clin Lab Sci 49:457–467
Rani P et al (2020) Clinicopathological correlation of tumor-stroma ratio and inflammatory cell infiltrate with tumor grade and lymph node metastasis in squamous cell carcinoma of buccal mucosa and tongue in 41 cases with review of literature. J Cancer Res Ther 16:445–451
Rashid M et al (2021) Up-down regulation of HIF-1α in cancer progression. Gene 798:145796
Sano D, Myers JN (2007) Metastasis of squamous cell carcinoma of the oral tongue. Cancer Metastasis Rev 26:645–662
Semenza GL (2007) Hypoxia-inducible factor 1 (HIF-1) pathway. Sci STKE. https://doi.org/10.1126/stke.4072007cm8
Shash LS, Ibrahim RA, Elgohary SA (2021) E-cadherin and N-cadherin immunohistochemical expression in proliferating urothelial lesions: potential novel cancer predictive EMT profiles. Appl Immunohistochem Mol Morphol 29:657–666
Siu A et al (2013) Expression of EMMPRIN modulates mediators of tumor invasion in oral squamous cell carcinoma. J Calif Dent Assoc 41:831–838
Sugita S, Yamato M, Hatabu T, Kataoka Y (2021) Involvement of cancer-derived EMT cells in the accumulation of (18)F-fluorodeoxyglucose in the hypoxic cancer microenvironment. Sci Rep 11:9668
Tirpe AA et al (2019) Hypoxia: overview on hypoxia-mediated mechanisms with a focus on the role of HIF genes. Int J Mol Sci 20:6140
Wang B, Liu J, Zhong Z (2021a) Prediction of lymph node metastasis in oral tongue squamous cell carcinoma using the neutrophil-to-lymphocyte ratio and platelet-to-neutrophil ratio. J Clin Lab Anal 35:e23684
Wang X, Priya Veeraraghavan V, Krishna Mohan S, Lv F (2021b) Anticancer and immunomodulatory effect of rhaponticin on Benzo(a)Pyrene-induced lung carcinogenesis and induction of apoptosis in A549 cells. Saudi J Biol Sci 28:4522–4531
Wong SHM et al (2018) E-cadherin: Its dysregulation in carcinogenesis and clinical implications. Crit Rev Oncol Hematol 121:11–22
Zhang Y et al (2021) hCINAP is potentially a direct target gene of HIF-1 and is required for hypoxia-induced EMT and apoptosis in cervical cancer cells. Biochem Cell Biol 99:203–213
Zhong Z et al (2018) FNDC3B promotes epithelial-mesenchymal transition in tongue squamous cell carcinoma cells in a hypoxic microenvironment. Oncol Rep 39:1853–1859
Zhou P (2010) Traditional Chinese medicine. Comb Chem High Throughput Screen 13:836
Zhou X et al (2015) Effect of HIF-1α on biological activation of human tongue squamous cell carcinoma SCC-15 cells in vitro. Int J Oncol 46:2346–2354
Acknowledgements
This work was supported by the National Nature Science Foundation of China (grant number 81960891) and the traditional Chinese medicine research project from Health commission of Jiangxi Province (grant number 2019A319 and 2020A025).
Author information
Authors and Affiliations
Contributions
YW and XW conceived the project and designed the experiments. YW, XW, YS, WW, WH, JZ, and LJ performed the experiments and analyzed the data. LJ wrote and revised the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
Yuan Wu, Xiaowen Wan, Yisen Shao, Wei Wang, Wenquan Huang, Jiajun Zhu and Lin Jiang declare that he/she has no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
13273_2023_337_MOESM1_ESM.tif
Supplementary file1 (TIF 2902 KB) Supplementary Figure S1. Knockdown of HIF-1α counteracts the effect of rhaponticin on SCC-15 cells in a hypoxic environment. A: RT-qPCR assessment of the mRNA level of HIF-1α in SCC-15 cells transfected with si-NC or si-HIF-1α under hypoxia (1% O2) for 24h. B: Western blotting assessment of the protein level of HIF-1α in SCC-15 cells transfected with si-NC or si-HIF-1α under hypoxia (1% O2) for 24h. C: Cell scratch assay assessment of the migration ability of SCC-15 cells intervened with rhaponticin (10 μM) or/and transfected with si-HIF-1α under hypoxia (1% O2) for 24h. D: Transwell assay assessment of the invasion ability of SCC-15 cells intervened with rhaponticin or/and transfected with si-HIF-1α under hypoxia (1% O2) for 24h. E: Western blotting assessment of the E-cadherin and N-cadherin protein levels in SCC-15 cells intervened with rhaponticin (10 μM) or/and transfected with si-HIF-1α under hypoxia (1% O2) for 24h. n=3. Data are presented as the means ± SEM. p value was based on one-way analysis of variance. ***P<0.001 vs. si-NC group
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Wu, Y., Wan, X., Shao, Y. et al. Rhaponticin suppresses the hypoxia-induced factor-1 alpha-mediated aggressive phenotype of tongue squamous cell carcinoma. Mol. Cell. Toxicol. 20, 259–269 (2024). https://doi.org/10.1007/s13273-023-00337-2
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13273-023-00337-2