Abstract
Background
There is active research on developing materials for improving skin function. Eggshell membrane (ESM) is one such raw material that is consumed as a functional food to support skin health. However, studies on the mechanism of improvement of skin function on ingestion of ESM are still lacking.
Objectives
To explore this mechanism of action, we conducted an ultraviolet (UV) irradiation study on a SKH-1 hairless mouse model. Feeding ESM was found to improve skin moisture and reduce wrinkles during 12 weeks of UVB irradiation.
Results
Oral administration of ESM restored moisture in the dorsal skin tissue of mice. In addition, oral ingestion of ESM also reversed the increased transepidermal water loss and reduction of mRNA expression of hyaluronic synthases induced by UVB irradiation. Furthermore, UVB irradiation-induced collagen degradation was inhibited, and the expression of the collagenase MMP was reduced in the ESM intake group compared to the control. These results confirmed that oral ingestion of the ESM has an anti-wrinkle effect. In addition, the mRNA expression of the antioxidant enzyme SOD1, which was reduced on UVB irradiation, was restored on ingestion of the ESM. Restoring the expression of antioxidant enzymes is a key strategy for improving skin function of the ESM.
Conclusion
Taken together, the findings from our study reveal the potential of ESM as a nutricosmetic material with anti-wrinkle and skin moisturizing properties.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Skin is the largest externally exposed organ in the human body, and undergoes several physiological changes in response to various environmental factors. Solar ultraviolet (UV) rays represent the major factor causing aberrant changes in skin tissue (Ferrara et al. 2020). In addition, solar UV irradiation can induce pre-maturation of human skin, also known as photoaging (Wlaschek et al. 2001). Increased wrinkle formation, skin dryness, acute erythema, and pigmentary changes have been suggested as hallmarks of UV-induced skin aging (Berry et al. 2019; Gromkowska-Kepka et al. 2021; Biniek et al. 2012). Solar UV radiation is composed of three wavelength ranges: UVA (320–400 nm), UVB (280–320 nm), UVC (100–280 nm). Although the ozone layer absorbs UVC in the atmosphere, UVA and UVB can reach the skin surface (Matsumura et al. 2004).
UVB irradiation can induce skin thickening and wrinkle formation by activating various intracellular signaling pathways such as MAPK, NF-κB, and PI3K/Akt in human keratinocytes (Oh et al. 2017; Terazawa et al. 2015). The MAPK signaling pathway is composed of the signaling molecules ERK, p38, and JNK, which regulate cellular proliferation, differentiation, development, and apoptosis (Zhang et al. 2002; Kim 2022). The MAPK signaling pathway also induces the transcription factor AP-1, which subsequently binds to the promoter region of MMP-1 (matrix metalloproteinases-1) gene (Vincenti et al. 2002) and induces the production and extracellular secretion of MMP-1 (Lu et al. 2016). MMPs are also produced in essentially all cell types, including interstitial, vascular, epithelial, and inflammatory cells; however, both MMPs expression levels and patterns vary among cell types and organs (Parks 2006). Particularly in the skin, MMP-1 expression causes fibrous collagen degradation in the extracellular matrix (ECM) (Ryu et al. 2018). Fibrous collagen is the main structural protein in the ECM of connective tissue. Among the various types of fibrous collagen, collagen type 1 accounts for 80–90% of skin collagen (Reilly et al. 2021), while collagen type 3 constitutes approximately 20% of adult skin (Weedon 2010). The procollagen a1 and a2 genes are first transcribed to form pre-pro-collagen, which then undergoes processing in the endoplasmic reticulum through the removal of a single peptide at its N-terminus, leading to the formation of procollagen (Wu et al. 2022). Loss of procollagen is a marker of photoaging, and its expression levels in human fibroblasts are reduced on UVB irradiation (Quan et al. 2004).
Chronic UVB irradiation induces skin dryness by attenuating hyaluronic acid (HA) content in the skin (Dai et al. 2007). HA is considered critical to skin hydration (Papakonstantinou et al. 2012) because of its water-holding capacity. HA is synthesized by hyaluronic acid synthase (HAS) enzymes (Sze et al. 2016; Dovedytis et al. 2020). The isoenzymes HAS1–3 produce HA chains of varying length (Stridh et al. 2012). HAS1 and HAS2 produce HA polymers up to 2 MDa in size, whereas HAS3 synthesizes HA polymers up to 1 MDa (Stridh et al. 2012). The three HAS isoforms share a high degree of homology (55–71%), and HAS2 is the most abundantly expressed form in keratinocytes (Marunaka et al. 2022). Photo exposure causes exogenous skin aging through changes in HA homeostasis (Papakonstantinou et al. 2012). In addition, UVB irradiation has been reported to decrease the expression of HAS2 (Kim et al. 2021) and downregulate HA synthesis (Kurdykowski et al. 2011). HA present in both the epidermis and dermis binds to the ECM through the CD44 receptor, subsequently improving epidermal barrier function and skin hydration (Wertz 2004). The epidermal barrier is essential for maintaining water content and balance (Rosso et al. 2016). UVB irradiation-induced degradation of the epidermal barrier decreases skin hydration (Li et al. 2022; Choi et al. 2021), and increases transepidermal water loss (TEWL) (Li et al. 2022). Thus, UVB irradiation induces skin dryness through various biological and physiological factors, which results in the release of proinflammatory cytokines from the disrupted skin barrier (Hänel et al. 2013).
UV irradiation can also induce the generation of reactive oxygen species (ROS) in the skin (Jin et al. 2007). ROS are highly reactive molecules that can damage cell structures such as nucleic acids, lipids, and carbohydrates (Birben et al. 2012). Physiologically, aberrant increases in ROS production have been demonstrated to be associated with various disorders, including premature aging, inflammation, skin dryness, and skin cancer (Ichihashi et al. 2003; Ahn et al. 2022). Intracellular ROS are sustained at a certain level by both enzymatic and non-enzymatic antioxidant systems present in the cell (Nimse et al. 2015). Superoxide dismutase (SOD), glutathione peroxidase (GPx), and catalase (CAT) are the major antioxidant enzymes (Nimse et al. 2015). ROS generation also induces the production of proinflammatory cytokines such as tumor necrosis factor-α (TNF-α), interleukin-1β (IL-1β) and interferon-γ (IFN-γ) (Yang et al. 2007). Taken together, irregular ROS generation induced by specific stimulators such as UVB exposure may cause several skin disorders (Padgett et al. 2013).
Eggshells protect their contents from mechanical damage and external contaminants (Tsai et al. 2006). The ESM comprises three layers: outer shell membrane, inner shell membrane, and limiting membrane (Shi et al. 2021). Protein fiber-bound calcium carbonate crystals form a matrix in the lamellar and spongy layers (Tsai et al. 2006). Importantly, the ESM has been demonstrated to exhibit anti-wrinkling and anti-inflammatory activities in vitro in a previous study. (Yoo et al. 2014). Additionally, a previous clinical study reported beneficial effects of oral administration of ESM on the skin, hair, and nails (Kalman et al. 2020). Other clinical trials have also indicated the improvement of connective tissue disorders on treatment with ESM (Ruff et al. 2009). Although the potential of the ESM as a nutricosmetic ingredient has been studied, the mechanism underlying the action of the ESM remains unclear.
Herein, we suggest the utility of ESM as a novel nutricosmetic ingredient. In our study, oral administration of ESM improved UVB-induced wrinkle formation and dryness in skin. Furthermore, UVB-induced proinflammatory cytokines were reduced and MAPK signaling pathways downregulated in the oral administration group compared to the control. We also confirmed the restoration of SOD1 mRNA expression in UVB-induced dorsal skin following ESM administration. As oxidative stress induced by UVB has been considered a significant factor for skin aging, oral administration of ESM could upregulate antioxidant systems in and confer anti-aging effect on skin.
Materials and methods
Reagent
Dulbecco's modified Eagle’s medium (DMEM) was purchased from Hyclone™. PBS and penicillin–streptomycin-neomycin (PSN) were obtained from GenDEPOT (Baker, TX, USA) and Sigma-Aldrich® (Burlington, MA, USA), respectively. Specific primary antibodies against p-MEK1/2, p-MEK3/6, p-MEK4, p-p38, p-JNK, and MMP-1 were obtained from Cell Signaling Technology (Danvers, MA, USA) and Biorbyt (Cambridge, UK). Vinculin and p-ERK were purchased from Santa Cruz Biotechnology® (Dallas, TX, USA) and R&D Systems (Minneapolis, MN, USA), respectively.
Eggshell membrane preparation
ESM was provided by Eggnovo S.L. (Spain). The eggshell was washed with water and separated into the shell and the ESM. The separated ESM was dried with hot air and sterilized, and crushed to small pieces < 30 µm in size. This preparation was referred to as the ESM (Ovoderm®-AS).
Animal study
The animal study protocol was reviewed and approved by the Institutional Animal Care and Use Committee of the Korea Food Research Institute (approval number: KFRI-M-21016). Ten mice from each group of 5-week-old female SKH-1 hairless mice were housed under appropriate condition (temperature: 23 ± 2, relative humidity: 50 ± 10%, 12 h light/dark cycle). All mice were freely provided with standard diet (AIN-93G) and drinking water. As described in Fig. 1A, after acclimatization for 1 week, the mice were UVB-irradiated and ingested ESM for 12 weeks. Ingestion of the ESM and UVB irradiation were performed thrice per week. UVB irradiation was started at a dose of 1 minimal erythema dose (MED) (0.05 J/cm2) and gradually increased to 4 MED at week 4. ESM was administered at 30, 60, and 120 mg/kg body weight. Body weight, food intake, TEWL, and skin moisture were measured after 12 weeks. TEWL was measured using a Tewameter® TM 300 (Courage & Khazaka Electronics, Cologne, Germany) and skin moisture was measured using a Corneometer CM825 (Courage & Khazaka Electronics).
It shows that animal study design, the ESM processed and safety of ESM administration. Animal study was experimented for 12 weeks with SKH-1 hairless female mouse. Ingestion of the ESM and UVB irradiation conduced for 12 weeks (A). The ESM was processed washing-separating-drying and sterilization-crushing (B). Intake safety of the ESM was evaluated by body weight (C) and food intake (n = 10) (D)
Dorsal skin wrinkle measurement
Silicon replicas (R201, Biobridge, Gyeonggi-do, Korea) were used to measure dorsal skin wrinkles in each mouse. Skin roughness was analyzed using the Visioscan VC98 (Courage & Khazaka Electronics), and the skin wrinkle depth was analyzed using ImageJ software with SurfCharJ plugin.
Histological analysis
After sacrificing the mice, the expression of specific proteins in the dorsal skin tissue was estimated using histological analysis. Briefly, mice dorsal skin tissues were fixed overnight with 10% formalin at 4 °C, and embedded in paraffin. Epidermal thickness was measured using hematoxylin and eosin (H&E) staining. Masson’s trichrome staining and immunohistochemistry of skin tissue were performed for collagen and MMP-1 staining. Dorsal skin tissues were observed under a microscope (20 × 40 magnification) (Nikon, Minato City, Japan). Intensity quantification and epidermal thickness were measured using ImageJ software.
Protein extraction and western blot
Protein was extracted from dorsal skin tissues of mice in lysis buffer (20 mM Tris–HCl [pH 7.5], 150 mM NaCl, 1 mM Na2EDTA, 1 mM EGTA, 1% Triton, 2.5 mM sodium pyrophosphate, 1 mM beta-glycerophosphate, 1 mM Na3VO4, and 1 µg/mL leupeptin) (Cell Signaling Technology®, Dallas, TX, USA). The solution was centrifuged at 18,956 ×g for 30 min at 4 °C, and protein concentration in the supernatant was estimated using the Pierce™ BCA Protein Assay Kit (23225, Thermo Fisher Scientific, MA, USA). The protein samples were then subjected to sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE). The separated proteins were transferred from the gel to PVDF membrane using Western blotting. The membranes were incubated with the indicated antibodies at 4 °C for 24 h following the manufacturer's instructions. Protein bands were detected using a chemiluminescence reader (LuminoGraph 3 Lite; Atto, Tokyo, Japan).
RNA extraction and quantitative real-time PCR
Total RNA was extracted from mouse dorsal skin tissues using TRIzol reagent (15596026; Thermo Fisher Scientific, Waltham, MA, USA). Complementary DNA was synthesized using amfiRivert cDNA synthesis platinum master mix (R5600-200, GenDEPOT, Katy, TX, USA) in a total reaction volume of 20 μL for 39 cycles. Quantitative PCR was conducted using Accupower® 2X GreenStar™ qPCR Master Mix (K-6253, Bioneer, Daejeon, Korea) on a CFX96 Touch Real-Time PCR Detection system (Bio-rad, Hercules, CA, USA). Primer sequences are listed in Table 1.
Quantification of hyaluronic acid (HA) and procollagen type 1
HA and procollagen type I were quantified using the HA quantitative test kit (HAE-163, Corgenix, CO, USA) and procollagen type 1 ELISA kit (AI72204A, Takara, Shiga, Japan), respectively, according to manufacturer's instructions.
Statistical analysis
All statistical analyses were performed using the SPSS software (version 20.0; SPSS Inc., Chicago, IL, USA). Data are expressed as mean ± standard deviation (SD). Statistical significance was determined using Student’s t test for single statistical comparisons, and p values < 0.05 were regarded significant.
Results
Oral administration of eggshell membrane prevents UVB-induced skin dryness
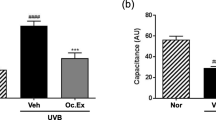
The manufacturing process of ESM is described in the Materials and methods section (Fig. 1B). As shown in Fig. 1C, D, ESM did not affect the changes in body weight and food intake of experimental animals. We measured the skin moisture content and transepidermal water loss (TEWL) in an animal study to investigate the effect of ESM intake on UVB-induced skin dryness. We found that repetitive exposure to UVB substantially reduced the moisture content in the dorsal skin of the mice. The skin moisture content was restored in a dose-dependent manner in the ESM administration group (Fig. 2A). Furthermore, we confirmed that TEWL, one of the indicators related to skin moisture, was increased by more than two-fold on UVB irradiation. Similar to the restoration of skin moisture content shown in Fig. 2A, TEWL was decreased on administration of the ESM (Fig. 2B). To evaluate the effect of the ESM on the expression of specific proteins related to skin hydration, we evaluated HAS1/2/3 mRNA expression in the dorsal skin of mice. Interestingly, UVB exposure dramatically diminished HAS1, HAS2, and HAS3 mRNA expression, and oral administration of the ESM reversed these reductions (Fig. 2C).
Oral ingestion of the ESM inhibits skin dryness. The effect of skin hydration and reduction of TEWL through oral ingestion of the ESM were evaluated at 12 weeks (A, B). Hyaluronic synthase (HAS) 1, 2, 3 were analyzed through mRNA of mouse dorsal skin tissues in quantitative PCR after sacrifice C. #, P < 0.05; ##, P < 0.01; significant differences between un-treated control and only UVB-treated group and *, P < 0.05; **, P < 0.01; *** P < 0.001; significant differences between UVB and UVB + ESM administration group
Oral administration of eggshell membrane reduces the deterioration of collagen production induced by UVB exposure
Next, we examined the effect of ESM administration on collagen production process, which is damaged on UVB exposure. The collagen content in the skin tissue was evaluated using Masson's trichrome staining, as described in the Materials and Methods. As shown in Fig. 3A, we observed a dramatic reduction in collagen content in UVB-exposed skin, accompanied by dose-dependent recovery of skin collagen content in ESM-administered mice. During collagen formation, procollagen is first endogenously produced from the encoding DNA and secreted into the extracellular space (Canty-Laird et al. 2005). The secreted procollagen molecules then self-assemble to generate mature collagen fibers (Canty-Laird et al. 2005). Therefore, we evaluated the effect of oral administration of ESM on procollagen production. Notably, we observed an attenuation of procollagen production in the UVB-exposed skin. In addition, this reduction in procollagen production was normalized in the ESM intake group (Fig. 3B). To confirm whether oral intake of ESM affects mature collagen content in the skin, we measured COL1A1 and COL3A1 mRNA expression, as described in Materials and methods. As expected, the mRNA expression of COL1A1 and COL3A1 was strikingly attenuated in the UVB-treated group, and restored with oral intake of the ESM (Fig. 3C). These results suggest that the oral intake of ESM confers anti-wrinkle effect against UVB-induced skin aging.
Oral administration of the ESM inhibits UVB irradiation-induced collagen degradation. In mouse dorsal skin tissues, collagen intensity was analyzed by masson’s trichrome staining (A). Lysates of mice dorsal skin tissues were analyzed procollagen (B) through ELISA and COL1A1 and COL3A1 mRNA through quantitative PCR (C). #, P < 0.05; ##, P < 0.01; ###, P < 0.001; significant differences between un-treated control and only UVB-treated group and *, P < 0.05; **, P < 0.01; *** P < 0.001; significant differences between UVB and UVB + ESM administration group
Oral administration of eggshell membrane attenuates fiber-degrading enzymes and wrinkle formation
In addition to procollagen expression, collagen degradation is modulated by UV exposure (Quan et al. 2004). Therefore, we investigated the levels of enzymes MMP-1 and MMP-2, which are involved in for the degradation of fiber components in the skin tissue. Similar to previous studies (Jung et al. 2014), the expression of MMP-1, a collagen-degrading enzyme, was highly upregulated in the UVB-exposed dorsal skin of mice in this study. Furthermore, downregulation of MMP-1 expression was observed in the ESM-administered group (Fig. 4A). Next, we examined the effect of the ESM on the gelatin-degrading enzyme MMP-2. As shown in Fig. 4B, MMP-2 expression was reduced in the ESM-treated group. Oral ingestion of ESM also reversed the UVB irradiation-induced increase in epidermal thickness (Fig. 4C) and wrinkle formation (Fig. 4D). These results indicate that ESM administration leads to the normalization of skin collagen content and inhibition of wrinkles through the suppression of production of fiber component-degrading enzymes.
Oral administration of the ESM reduces UVB irradiation-induced MMP expression and wrinkle formation. MMP-1 expression in dorsal skin tissues was analyzed in immunohistochemistry (A). MMP-2 mRNA expression in mouse dorsal skin tissues was evaluated by quantitative PCR (B). The epidermis thickness was measured by imageJ in H&E staining (C). Skin roughness was evaluated in dorsal skin replica and relative wrinkle depth was measured by imageJ on the replica (D). ##, P < 0.01; ###, P < 0.001; significant differences between un-treated control and only UVB-treated group and **, P < 0.01; *** P < 0.001; significant differences between UVB and UVB + ESM administration group
Oral administration of eggshell membrane reduces UVB-induced cytokine expression through upregulation of SOD1
Excessive UVB exposure is known to induce immune hyperactivation through aberrant oxidative stress (Yang et al. 2007). Therefore, we analyzed the degree of cytokine expression in the dorsal skin of the mice. As shown in Fig. 5A, UVB irradiation increased the mRNA expression of cytokines such as IL-1, IL-6, and TNF-α, which are related to skin inflammation (Hänel et al. 2013). In addition, oral administration of ESM reversed the UVB-induced elevation in cytokine expression (Fig. 5A), and reduced the mRNA expression of cyclooxygenase-2 (COX-2), which is the enzyme responsible for causing inflammation (Carola et al. 2021). Next, we investigated the expression levels of antioxidant enzymes to confirm their relation to the suppression of cytokine expression in the ESM-treated group. Notably, we found that UVB treatment dramatically reduced SOD1 expression, and dose-dependent recovery was observed on oral administration of ESM (Fig. 5B). Overall, we suggest that oral administration of ESM suppresses UVB-induced cytokine expression via upregulation of the antioxidant enzyme SOD1.
Oral intake of the ESM suppresses cytokine production and restores the expression of antioxidant enzymes. The mRNA in mouse dorsal skin tissues was extracted by trizol, and cytokines and antioxidant enzyme SOD1 were analyzed in quantitative PCR (A), (B). #, P < 0.05; ###, P < 0.001; significant differences between un-treated control and only UVB-treated group and *, P < 0.05; **, P < 0.01; *** P < 0.001; significant differences between UVB and UVB + ESM administration group
Oral administration of eggshell membrane inhibits the UVB-induced MEK1/2-ERK and MEK4-JNK but not MEK3/6-p38 signaling pathway
The MAPK signaling pathways including ERK, p38, and JNK that upregulate MMP1 expression are phosphorylated by UVB irradiation (Nelson et al. 2004). We investigated whether oral ingestion of the ESM affects the MEK1/2-ERK and MEK4-JNK signaling pathways. As shown in Fig. 6A, UVB irradiation substantially induced phosphorylation of MEK1/2 and MEK4, and decreased phosphorylation levels were observed in the ESM oral intake group compared to the control. Subsequently, the phosphorylation of ERK and JNK, the downstream signaling molecules of MEK1/2 and MEK4, respectively, was dramatically attenuated in the ESM oral administration group (Fig. 6B). Interestingly, the MEK3/6-p38 pathway was not affected by oral ingestion of the ESM (Fig. 6A, B). Regardless, these results indicate that ESM administration abolishes UVB-induced skin damage by attenuating the MEK1/2-ERK and MEK4-JNK signaling pathways stimulated by UVB.
Oral intake of eggshell membrane modulates specific kinases. The protein lysates of mouse dorsal skin tissues were analyzed by western blot. The intensity of protein band is quantified by imageJ. #, P < 0.05; significant differences between un-treated control and only UVB-treated group and *, P < 0.05; **, P < 0.01; significant differences between UVB and UVB + ESM administration group
Discussion
Yoo et al. reported the anti-wrinkle, anti-inflammatory, and antimicrobial activities of ESM hydrolysates (Yoo et al. 2014). Furthermore, the effects of oral ingestion of ESM on skin aging and differentiation have been studied (Furukawa et al. 2021). However, the mechanism underlying the anti-aging effect of ESM on the skin was not clearly identified in these studies.
The skin is the main barrier between the body and environmental factors (Parrado et al. 2019). Although solar UV radiation exerts several positive effects on human skin, such as vitamin D production, chronic and excessive UV exposure can cause various skin disorders such as photoaging, inflammation, and carcinogenesis (Bosch et al. 2015).
It is well established that UVB irradiation induces intracellular ROS generation (Heck et al. 2003). ROS produced through mitochondrial metabolism causes skin diseases, such as wrinkle formation, skin dryness, and inflammation (Murphy 2009; Rinnerthaler et al. 2015). The ROS generated on UVB irradiation primarily induce the secretion of cytokines, such as TNF-α, IL-6, and IL-1β (Padgett et al. 2013). Herein, we confirmed that the production and secretion of these cytokines were inhibited by the oral ingestion of ESM (Fig. 5A). UVB irradiation-induced ROS generation also leads to a decrease in antioxidant enzyme levels and activates the MAPK signaling pathway, which upregulates MMPs (Nelson et al. 2004; Wang et al. 2019). Consequently, the increased expression of MMPs promotes wrinkle formation (Kim et al. 2013). Furthermore, overproduction of ROS was also shown to decrease procollagen expression (Zhang et al. 2017). We found that oral intake of ESM recovered the expression of the antioxidant enzyme SOD1 (Fig. 5B), and consequently attenuated the UVB-induced upregulation of the MAPK signaling pathway and the associated cytokine production (Figs. 5A, 6A, B). With regard to the mechanism behind the action of ESM, the phosphorylation of MEK1/2-ERK and MEK4-JNK pathway components was found to be specifically attenuated on oral administration of ESM, although MEK3/6-p38 phosphorylation was not affected (Fig. 5A, B, S1). Interestingly, several studies reported that some MAP3 kinase family including Raf, Mos, Tpl, and MUK were observed to specifically phosphorylate the MEK1/2-ERK and pMEK4-JNK pathways, but not MEK3/6-p38 (Raman et al. 2007). Therefore, we hypothesized that oral administration of the ESM may target the above-mentioned kinases to regulate the MEK1/2-ERK and pMEK4-JNK pathways. Taken together, these findings suggest that SOD1 restored through ESM administration reduces anti-wrinkle formation by reducing MMP-1 and improving procollagen expression (Figs. 3A, 4A, D).
Repeated exposure to UV irradiation is associated with skin dryness (Kang et al. 2019). UV irradiation has been reported to downregulate HAS1, 2, 3 mRNA expression (Park et al. 2017). In particular, HAS2 is essential for the maintenance of skin hydration. The decrease in HAS levels and skin moisture due to UVB irradiation was recovered in the orally ingested ESM group in this study (Fig. 2A, C). In addition, the increase in TEWL was attenuated on ESM administration (Fig. 2B). Furthermore, ROS, which break down HA, are also associated with skin hydration (Soltés et al. 2006). We propose that the restoration of HAS expression on oral ingestion of ESM is important for suppressing skin dryness. Taken together, ESM can utilize to as an anti-aging nutricosmetic material. Based on these findings, there are necessary to identify the target molecule using multi-omic analysis. Moreover, both safety evaluation in vivo test and anti-aging effect in clinical trial of ESM are also require for developing nutricosmetic material.
Conclusion
Antioxidant activity in the human body is associated with both enzymatic and non-enzymatic systems. This study confirmed that the oral ingestion of ESM restored the antioxidant enzyme SOD1 levels, which were reduced on UVB irradiation. This suppressed the activity of the MAPK-MMP signaling system and restored procollagen expression. Furthermore, oral intake of ESM also repaired the UV-induced decreased in HAS expression, leading to restoration of skin moisturizing ability. In addition, it also inhibited the production of cytokines. Thus, the findings from our study indicate the potential of ESM for use as a nutricosmetic material, which has an anti-wrinkle formation effect and improves skin hydration.
References
Ahn Y et al (2022) Photoprotective effects of sphingomyelin-containing milk phospholipids in ultraviolet B-irradiated hairless mice by suppressing nuclear factor-κB expression. J Dairy Sci 105:1929–1939
Berry CW et al (2019) Skin erythema and blood flow responses to acute ultraviolet radiation exposure. FASEB J 33:541–541
Biniek K, Levi K, Dauskardt RH (2012) Solar UV radiation reduces the barrier function of human skin. P Natl Acad Sci USA 109:17111–17116
Birben E et al (2012) Oxidative stress and antioxidant defense. World Allergy Organ J 5:9–19
Bosch R et al (2015) Mechanisms of photoaging and cutaneous photocarcinogenesis, and photoprotective strategies with phytochemicals. Antioxid (basel) 4:248–268
Canty-Laird E, Kadler K (2005) Procollagen trafficking, processing and fibrillogenesis. J Cell Sci 118:1341–1353
Carola C et al (2021) A cornflower extract containing N-Feruloylserotonin reduces inflammation in human skin by neutralizing CCL17 and CCL22 and inhibiting COX-2 and 5-LOX. Mediators Inflamm 2021:6652791
Choi SI et al (2021) Eisenia bicyclis extract repairs UVB-induced skin photoaging in vitro and in vivo: photoprotective effects. Mar Drugs 19:693–693
Dai G et al (2007) Chronic ultraviolet B irradiation causes loss of hyaluronic acid from mouse dermis because of down-regulation of hyaluronic acid synthases. Am J Pathol 171:1451–1461
Dovedytis M, Liu ZJ, Bartlett S (2020) Hyaluronic acid and its biomedical applications: a review. Eng Regen 1:102–113
Ferrara F et al (2020) Check updates additive effect of combined pollutants to UV induced skin oxinflammation damage evaluating the protective topical application of a cosmeceutical mixture formulation. Redox Biol 34:101481
Furukawa K et al (2021) Effects of eggshell membrane on keratinocyte differentiation and skin aging in vitro and in vivo. Nutrients 13:2144–2144
Gromkowska-Kepka KJ, Puscion-Jakubik A, Markiewicz-Zukowska R, Socha K (2021) The impact of ultraviolet radiation on skin photoaging—review of in vitro studies. J Cosmet Dermatol 20:3427–3431
Hänel KH, Cornelissen C, Lüscher B, Baron JM (2013) Cytokines and the skin barrier. Int J Mol Sci 14:6720–6745
Heck DE, Vetrano AM, Mariano TM, Laskin JD (2003) UVB light stimulates production of reactive oxygen species: unexpected role for catalase. J Biol Chem 278:22432–22436
Ichihashi M et al (2003) UV-induced skin damage. Toxicology 189:21–39
Jin GH et al (2007) UVB induced oxidative stress in human keratinocytes and protective effect of antioxidant agents. Radiat Environ Bioph 46:61–68
Jung HY et al (2014) Pinus densiflora extract protects human skin fibroblasts against UVB-induced photoaging by inhibiting the expression of MMPs and increasing type I procollagen expression. Toxicol Rep 1:658–666
Kalman DS, Hewlings S (2020) The effect of oral hydrolyzed eggshell membrane on the appearance of hair, skin, and nails in healthy middle-aged adults: a randomized double-blind placebo-controlled clinical trial. J Cosmetic Dermatol 19:1463–1472
Kang W, Choi D, Park T (2019) Dietary suberic acid protects against uvb-induced skin photoaging in hairless mice. Nutrients 11:2948–2948
Kim SR et al (2013) Anti-wrinkle and Anti-inflammatory effects of active garlic components and the inhibition of MMPs via NF-κB signaling. PLoS ONE 8:e73877
Kim JH (2022) Hyaluronic acid suppresses the effect of di-(2-ethylhexyl) phthalate in HaCaT keratinocytes. Mol Cellular Toxicol 18:549–556
Kim SH et al. (2021) Aqueous extract of Phragmites communis rhizomes attenuates phototoxicity in skin cells (Nov, https://doi.org/10.1007/s13273-020-00106-5, 2020). Mol Cellular Toxicol 17:91–91
Kurdykowski S et al (2011) Ultraviolet-B irradiation induces differential regulations of hyaluronidase expression and activity in normal human keratinocytes. Photochem Photobiol 87:1105–1112
Li ZZ et al (2022) Ginsenosides repair UVB-induced skin barrier damage in BALB/c hairless mice and HaCaT keratinocytes. J Ginseng Res 46:115–125
Lu J et al (2016) Tiron inhibits UVB-induced AP-1 binding sites transcriptional activation on MMP-1 and MMP-3 promoters by MAPK signaling pathway in human dermal fibroblasts. PLoS ONE 11:e0159998
Marunaka K et al (2022) Elevation of hyaluronan synthase by magnesium supplementation mediated through the activation of GSK3 and CREB in human keratinocyte-derived HaCaT cells. Int J Mol Sci 23:71
Matsumura Y, Ananthaswamy HN (2004) Toxic effects of ultraviolet radiation on the skin. Toxicol Appl Pharmacol 195:298–308
Murphy MP (2009) How mitochondria produce reactive oxygen species. Biochem J 417:1–13
Nelson KK, Melendez JA (2004) Mitochondrial redox control of matrix metalloproteinases. Free Radic Biol Med 37:768–784
Nimse SB, Pal D (2015) Free radicals, natural antioxidants, and their reaction mechanisms. Rsc Adv 5:27986–28006
Oh YR et al (2017) Ginsenoside Rc protects against UVB-induced photooxidative damage in epidermal keratinocytes. Mol Med Rep 16:2907–2914
Padgett LE et al (2013) The role of reactive oxygen species and proinflammatory cytokines in type 1 diabetes pathogenesis. Ann NY Acad Sci 1281:16–35
Papakonstantinou E, Roth M, Karakiulakis G (2012) Hyaluronic acid: a key molecule in skin aging. Dermatoendocrinol 4:253–258
Park K et al (2017) Cactus cladodes (Opuntia humifusa) extract minimizes the effects of UV irradiation on keratinocytes and hairless mice. Pharm Biol 55:1032–1040
Parks WC (2006) Matrix metalloproteinases. In: Laurent GJ, Shapiro SD (eds) Encyclopedia of respiratory medicine. Academic Press, Oxford, pp 18–25
Parrado C et al (2019) Environmental stressors on skin aging mechanistic insights. Front Pharm 10:759–759
Quan T et al (2004) Solar ultraviolet irradiation reduces collagen in photoaged human skin by blocking transforming growth factor-beta type II receptor/Smad signaling. Am J Pathol 165:741–751
Raman M, Chen W, Cobb MH (2007) Differential regulation and properties of MAPKs. Oncogene 26:3100–3112
Reilly DM, Lozano J (2021) Skin collagen through the lifestages: importance for skin health and beauty. Plastic Aesthetic Res 8:2–2
Rinnerthaler M et al (2015) Oxidative stress in aging human skin. Biomolecules 5:545–589
Rosso JD et al (2016) Understanding the epidermal barrier in healthy and compromised skin: clinically relevant information for the dermatology practitioner: proceedings of an expert panel roundtable meeting. J Clin Aesthet Dermatol 9:S2–S8
Ruff KJ, DeVore DP, Leu MD, Robinson MA (2009) Eggshell membrane: a possible new natural therapeutic for joint and connective tissue disorders. Results from two open-label human clinical studies. Clin Interv Aging 4:235–240
Ryu JY, Na EJ (2018) MMP expression alteration and MMP-1 production control by syringic acid via AP-1 mechanism. Biomed Dermatol 2:15–15
Shi YN et al (2021) Avian eggshell membrane as a novel biomaterial: a review. Foods 10:2178–2178
Soltés L et al (2006) Degradative action of reactive oxygen species on hyaluronan. Biomacromol 7:659–668
Stridh S, Palm F, Hansell P (2012) Renal interstitial hyaluronan: functional aspects during normal and pathological conditions. Am J Physiol Regul Integr Comp Physiol 302:R1235–R1249
Sze JH, Brownlie JC, Love CA (2016) Biotechnological production of hyaluronic acid: a mini review. 3 Biotech 6:67–67
Terazawa S et al (2015) The UVB-stimulated expression of transglutaminase 1 is mediated predominantly via the NFκB signaling pathway: new evidence of its significant attenuation through the specific interruption of the p38/MSK1/NFκBp65 Ser276 Axis. PLoS ONE 10:e0136311
Tsai WT et al (2006) Characterization and adsorption properties of eggshells and eggshell membrane. Bioresour Technol 97:488–493
Vincenti MP, Brinckerhoff CE (2002) Transcriptional regulation of collagenase (MMP-1, MMP-13) genes in arthritis: integration of complex signaling pathways for the recruitment of gene-specific transcription factors. Arthritis Res 4:157–164
Wang Y et al (2019) Protective skin aging effects of cherry blossom extract (Prunus Yedoensis) on oxidative stress and apoptosis in UVB-irradiated HaCaT cells. Cytotechnology 71:475–487
Weedon D (2010) In disorders of collagen in weedon’s skin pathology, 3rd edn. Churchill Livingstone, Edinburgh
Wertz PW (2004) Stratum corneum lipids and water. Exog Dermatol 3:53–56
Wlaschek M et al (2001) Solar UV irradiation and dermal photoaging. J Photochem Photobiol B 63:41–51
Wu M, Cronin K, Crane JS (2022) In biochemistry, collagen synthesis. In StatPearls, Treasure Island (FL)
Yang D et al (2007) Pro-inflammatory cytokines increase reactive oxygen species through mitochondria and NADPH oxidase in cultured RPE cells. Exp Eye Res 85:462–472
Yoo J et al (2014) Effects of egg shell membrane hydrolysates on anti-inflammatory, anti-wrinkle, anti-microbial activity and moisture-protection. Korean J Food Sci Anim Resour 34:26–32
Zhang W, Liu HT (2002) MAPK signal pathways in the regulation of cell proliferation in mammalian cells. Cell Res 12:9–18
Zhang Q et al (2017) Excess mechanical stress and hydrogen peroxide remodel extracellular matrix of cultured human uterosacral ligament fibroblasts by disturbing the balance of MMPs/TIMPs via the regulation of TGFβ1 signaling pathway. Mol Med Rep 15:423–430
Acknowledgements
This research was supported by “Regional Innovation Strategy (RIS)” through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (MOE) (2021RIS-001) and was also supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (2022R1A6A1A03055869).
Author information
Authors and Affiliations
Contributions
W-JS, JA, EL and T-GL. designed research studies. W-JS, JA and WL conducted the experiments. WL, D JS and EL performed data analysis, provided reagents. All the authors participated in manuscript preparation and approved the final manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
Woo-Jin Sim declares that he has no conflict of interest. Jisong Ahn declares that she has no conflict of interest. Wonchul Lim declares that he has no conflict of interest. Dong Ju Son declares that he has no conflict of interest. Eunjung Lee declares that she has no conflict of interest. Tae-Gyu Lim declares that he has no conflict of interest.
Ethical approval
The animal study protocol was approved by the Institutional Review Board (or Ethics Committee) of Institutional Animal Care and Use Committee of the Korea Food Research Institute (protocol code KFRI-M-21016).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Sim, WJ., Ahn, J., Lim, W. et al. Anti-skin aging activity of eggshell membrane administration and its underlying mechanism. Mol. Cell. Toxicol. 19, 165–176 (2023). https://doi.org/10.1007/s13273-022-00291-5
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13273-022-00291-5