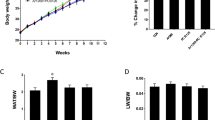
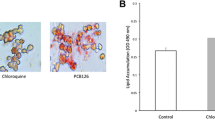
Abstract
Bisphenol A (BPA) is an environmental toxicant that causes adverse effects to liver even at low concentrations. Non-alcoholic fatty liver disease (NAFLD) presents as significant accumulation of lipids in the liver, impairing detoxification processes. Nevertheless, little information is available regarding the susceptibility of NAFLD to BPA toxicity. Here, we compared the effects of low concentration BPA exposure on the protein profiles of normal and NAFLD cells. We established NAFLD model by treating HepG2 cells with fatty acids and subsequently treated with low BPA concentration. Protein expression profiles showed that four proteins from normal cells and nine proteins from NAFLD cells were dysregulated after BPA treatment. A pathway analysis revealed decreased expression of translation elongation factor proteins in NAFLD cells following BPA treatment and this down-regulation was confirmed by immunoblotting. Our findings suggest that BPA toxicity is more deleterious, especially to translation elongation, in NAFLD cells than normal cells.
Similar content being viewed by others
References
U.S. Environmental Protecting Agency. Bisphenol A Action Plan. Retrieved July 5th, 2016, from the U.S. Environmental Protecting Agency Website: https:// www.epa.gov/assessing-andmanaging-chemicalsunder-tsca/bisphenol-bpa-action-plan (2010).
U.S. Food and Drug Administration. Update on Bisphenol A for Use in Food Contact Applications. Retrieved July 9th, 2016, from the U.S. Food and Drug Administration Website: http://www.fda.gov/News Events/PublicHealthFocus/ucm064437.htm (2010).
Bureau of Chemical Safety, Food Directorate Health Products and Food Branch. Survey of Bisphenol A in Canned Food Products from Canadian Markets. Authority of the Minister of Health, Ottawa, Ontario (2010).
Carwile, J. L. & Michels, K. B. Urinary bisphenol A and obesity: NHANES 2003-2006. Environ Res 111: 825–830 (2011).
Noonan, G. O., Ackerman, L. K. & Begley, T. H. Concentration of bisphenol A in highly consumed canned foods on the U.S. market. J Agric Food Chem 59: 7178–7185 (2011).
Huang, Y. Q. et al. Bisphenol A (BPA) in China: a review of sources, environmental levels, and potential human health impacts. Environ Int 42: 91–99 (2012).
Funabashi, T., Kawaguchi, M. & Kimura, F. The endocrine disruptors butyl benzyl phthalate and bisphenol A increase the expression of progesterone receptor messenger ribonucleic acid in the preoptic area of adult ovariectomized rats. Neuroendocrinology 74: 77–81 (2001).
Salian, S., Doshi, T. & Vanage, G. Neonatal exposure of male rats to bisphenol A impairs fertility and expression of Sertoli cell junctional proteins in the testis. Toxicology 265: 56–67 (2009).
Wu, J. H. et al. Oral exposure to low-dose bisphenol A aggravates testosterone-induced benign hyperplasia prostate in rats. Toxicol Ind Health 27: 810–819 (2011).
Tainaka, H. et al. Evaluation of the testicular toxicity of prenatal exposure to bisphenol A based on microarray analysis combined with MeSH annotation. J Toxicol Sci 37: 539–548 (2012).
Zhang, W. Z., Yong, L., Jia, X. D., Li, N. & Fan, Y. X. Combined subchronic toxicity of bisphenol A and dibutyl phthalate on male rats. Biomed Environ Sci 26: 63–69 (2013).
Somm, E. et al. Perinatal exposure to bisphenol A alters early adipogenesis in the rat. Environ Health Perspect 117: 1549–1555 (2009).
Hassan, Z. K. et al. Bisphenol A induces hepatotoxicity through oxidative stress in rat model. Oxid Med Cell Longev 2012: 194829 (2012).
Huc, L., Lemarie, A., Gueraud, F. & Helies-Toussaint, C. Low concentrations of bisphenol A induce lipid accumulation mediated by the production of reactive oxygen species in the mitochondria of HepG2 cells. Toxicol In Vitro 26: 709–717 (2012).
Shankar, A., Teppala, S. & Sabanayagam, C. Urinary bisphenol A levels and measures of obesity: results from the national health and nutrition examination survey 2003-2008. ISRN Endocrinol 2012: 965243 (2012).
Bradley, E. L., Read, W. A. & Castle, L. Investigation into the migration potential of coating materials from cookware products. Food Addit Contam 24: 326–335 (2007).
Silver, M. K., O’Neill, M. S., Sowers, M. R. & Park, S. K. Urinary bisphenol A and type-2 diabetes in U.S. adults: data from NHANES 2003-2008. PLoS One 6: e26868 (2011).
Shankar, A. & Teppala, S. Urinary bisphenol A and hypertension in a multiethnic sample of US adults. J Environ Public Health 2012: 481641 (2012).
Kovacic, P. How safe is bisphenol A? Fundamentals of toxicity: metabolism, electron transfer and oxidative stress. Med Hypotheses 75: 1–4 (2010).
Geens, T. et al. A review of dietary and non-dietary exposure to bisphenol-A. Food Chem Toxicol 50: 3725–3740 (2012).
Merrell, M. D. & Cherrington, N. J. Drug metabolism alterations in nonalcoholic fatty liver disease. Drug Metab Rev 43: 317–334 (2011).
LaBrecque, D. Abbas et al. Nonalcoholic Fatty Liver Disease and Nonalcoholic Steatohepatitis (World Gastroenterology Organisation Global Guidelines. Milwaukee, MI, 2012).
Younossi, Z. M. et al. Global epidemiology of nonalcoholic fatty liver disease - meta-analytic assessment of prevalence, incidence and outcomes. Hepatology 64: 73–84 (2016).
Welshons, W. V., Nagal, S. C. & vom Saal, F. Large effects from small exposure. III. Endocrine mechanisms mediating effects of bisphenol A at levels of human exposure. Endocrinology 147:S56–69 (2006).
Cao, X. et al. Background bisphenol A in experimental materials and its implication to low-dose in vitro study. Chemosphere 81: 817–820 (2010).
Chavez-Tapia, N. C., Rosso, N. & Tiribelli, C. Effect of intracellular lipid accumulation in a new model of non-alcoholic fatty liver disease. BMC Gastroenterology 12: doi:10.1186/1471-230X-12-20 (2012).
Cui, W., Chen, S. L. & Hu, K. Quantification and mechanisms of oleic acid-induced steatosis in HepG2 cells. Am J Transl Res 2: 95–104 (2010).
Garcia-Ruiz, I., Solis-Munoz, P., Fernandez-Moreira, D., Munoz-Yague, T. & Solis-Herruzo, J. A. In vitro treatment of HepG2 cells with saturated fatty acids reproduces mitochondrial dysfunction found in nonalcoholic steatohepatitis. Dis Model Mech 8: 183–191 (2015).
Tinaikos, D. G., Vos, M. B. & Brunt, E. M. Nonalcoholic fatty liver disease: pathology and pathogenesis. Annu Rev Pathol Mech Dis 5: 145–171 (2010).
Lake, A. D. et al. Analysis of global and absorption, distribution, metabolism and elimination gene expression in the progressive stages of human nonalcoholic fatty liver disease. Drug Metab Dispos 39: 1954–1960 (2011).
Yalcin, E. B., Kulkarni, S. R., Slitt, A. L. & King, R. Bisphenol A sulfonation is impaired in metabolic and liver disease. Toxicol Appl Pharmacol 292: 75–84 (2016).
Browne G. J. & Proud, C. G. Regulation of peptidechain elongation in mammalian cells. Eur J Biochem 269: 5360–5368 (2002).
Qiu, F. et al. Eukaryotic elongation factor-1α 2 knockdown inhibits hepatocarcinogenesis by suppressing PI3K/Akt/NF-κB signaling. World J Gastroenterol 22: 4226–4237 (2016).
Stoianov, A. M., Robson, D. L., Hetherington, A. M., Sawyez, C. G. & Borradaile, N. M. Elongation factor 1A-1 is a mediator if hepatocyte lipotoxicity partly through its canonical function in protein synthesis. PLoS One 10:e0131269 (2015).
Geldof, A. A., Mastbergen, S. C., Henrar, R. E. C. & Faircloth, G. T. Cytotoxicity and neurocytotoxicity of new marine anticancer agents evaluated using in vitro assays. Cancer Chemother Pharmacol 44: 312–318 (1999).
Kaul, G., Pattan, G. & Rafeequi, T. Eukaryotic elongation factor-2 (eEF2): its regulation and peptide chain elongation. Cell Biochem Funct 29: 227–234 (2011).
Nakamura, J. et al. Overexpression of eukaryotic elongation factor eEF2 in gastrointestinal cancers and its involvement in G2/M progression in the cell cycle. Int J Oncol 34: 1181–1189 (2009).
Lindqvist, L. et al. Inhibition of translation by cytotrienin A - a member of the ansamycin family. RNA 16: 2404–2413 (2010).
Zhong, J. et al. Transfer RNAs mediate the rapid adaptation of Escherichia coli to oxidative stress. PLoS Genet 11:e1005320 (2015).
Nakamura, M. et al. Phosphoproteomic profiling of human SH-SY5Y neuroblastoma cells during response to 6-hydroxydopamine-induced oxidative stress. Biochim Biophys Acta 1763: 977–989 (2006).
Parrado, J., Absi, E. H., Machado, A. & Ayala, A. “In vitro” effect of cumene hydroperoxide on hepatic elongation factor-2 and its protection by melatonin. Biochim Biophys Acta 1624: 139–144 (2003).
Arguelles, S., Machado, A. & Ayala, A. “In vitro” effect of lipid peroxidation metabolites on elongation factor-2. Biochim Biophys Acta 1760: 445–452 (2006).
Moon, M. K. et al. Bisphenol A impairs mitochondrial function in the liver at doses below the no observed adverse effect level. J Korean Med Sci 27: 644–652 (2012).
Boucher, J. G. et al. Identification of mechanisms of action of bisphenol A-induced human preadipocyte differentiation by transcriptional profiling. Obesity 22: 2333–2343 (2014).
GoodsonIII, W. H. et al. Activation of the mTOR pathway by low levels of xenoestrogens in breast epithelial cells from high-risk women. Carcinogenesis 32: 1724–1733 (2011).
Ribeiro-Varandas, E. et al. Bisphenol A disrupts transcription and decreases viability in aging vascular endothelial cells. Int J Mol Sci 15: 15791–15805 (2014).
Huang, K. & Fingar, D. C. Growing knowledge of the mTOR signaling network. Semin Cell Dev Biol 36: 79–90 (2014).
Parkhitko, A. A., Favorova, O. O., Khabibullin, D. I., Anisimov, V. N. & Henske, E. P. Kinase mTOR: Regulation and role in maintenance of cellular homeostasis, tumor development and aging. Biochemistry (Mosc) 79: 88–101 (2014).
Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J Immunol Methods 65: 55–63 (1983).
Reamtong, O. et al. Protein profiling of mefloquine resistant Plasmodium falciparum using mass spectrometry-based proteomics. Int J Mass Spectrom 391: 82–92 (2015).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Chienwichai, P., Topanurak, S., Reamtong, O. et al. Effects of low bisphenol A concentration on protein expression profiles in an in vitro model of non-alcoholic fatty liver disease. Mol. Cell. Toxicol. 14, 61–70 (2018). https://doi.org/10.1007/s13273-018-0008-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13273-018-0008-2