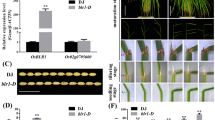
Abstract
Background
Brassinosteroids (BR) are essential growth hormone in plants. Various components involved in signal transduction pathway have been identified as targets of 14-3-3 phospho-binding proteins. Previously, we showed that 14-3-3 proteins directly interact with the Brassinosteroid Insensitive 1 (BRI1), the BR receptor kinase, and are also subject to phosphorylation in a BR-dependent manner.
Objective
In this study, we aimed to examine a potential interplay between 14-3-3 proteins and BRI1 in plant growth.
Methods
Morphological phenotypes of a T-DNA insertion mutant line, 14-3-3ψφε, defective in three 14-3-3 isoforms, psi, phi and epsilon, were characterized and compared with bri1-5 and two transgenic lines for BRI1, BRI1-Flag and BRI1-Flag (14-3-3ψφε). We also generated complementation lines carrying each of the three 14-3-3 genes and determined their differences in rosette growth.
Results
No significant differences between the wild-type and 14-3-3ψφε seedlings were observed regardless of BR applications. However, BRI1-Flag (14-3-3ψφε) showed a significantly reduced cold tolerance and BR sensitivity in hypocotyl and root development when compared to BRI1-Flag. In addition, narrower leaf shape and smaller rosette size were observed in BRI1-Flag (14-3-3ψφε), while the mutant phenotypes were partially restored in the complementation lines, two of which with 14-3-3φ and 14-3-3ε showed the rosette growth comparable to BRI1-Flag.
Conclusion
Taken together, our results suggested that 14-3-3 proteins might positively regulate BRI1 activity and showed that 14-3-3 isoforms have different functional impacts in BR signaling.



Similar content being viewed by others
References
Abarca D, Madueño F, Martı́nez-Zapater JM, Salinas J (1999) Dimerization of Arabidopsis 14-3-3 proteins: structural requirements within the N-terminal domain and effect of calcium. FEBS Lett 462:377–382
Aitken A, Collinge DB, Van Heusden BPH, Isobe T, Roseboom PH, Rosenfeld G, Soll J (1992) 14-3-3 proteins: a highly conserved, widespread family of eukaryotic proteins. Trends Biochem Sci 17:498–501
Bajguz A, Tretyn A (2003) The chemical characteristic and distribution of brassinosteroids in plants. Phytochemistry 62:1027–1046
Camoni L, Visconti S, Aducci P, Marra M (2018) 14-3-3 proteins in plant hormone signaling: doing several things at once. Front Plant Sci 9:297
Catalá R, López-Cobollo R, Castellano MM, Angosto T, Alonso JM, Ecker JR, Salinas J (2014) The Arabidopsis 14-3-3 protein RARE COLD INDUCIBLE 1A links low-temperature response and ethylene biosynthesis to regulate freezing tolerance and cold acclimation. Plant Cell 26:3326–3342
Chae WB, Park YJ, Lee KS, Nou IS, Oh MH (2016) Plant receptor kinases bind and phosphorylate 14-3-3 proteins. Genes Genom 38:1111–1119
Chang IF, Curran A, Woolsey R, Quilici D, Cushman JC, Mittler R, Harmon A, Harper JF (2009) Proteomic profiling of tandem affinity purified 14-3-3 protein complexes in Arabidopsis thaliana. Proteomics 9:2967–2985
Chevalier D, Morris ER, Walker JC (2009) 14-3-3 and FHA domains mediate phosphoprotein interactions. Annu Rev Plant Biol 60:67–91
Clough SJ, Bent AF (1998) Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J 16:735–743
Clouse SD, Sasse JM (1998) Brassinosteroids: essential regulators of plant growth and development. Annu Rev Plant Biol 49:427–451
de Zelicourt A, Colcombet J, Hirt H (2016) The role of MAPK modules and ABA during abiotic stress signaling. Trends Plant Sci 21:677–685
del Viso F, Casaretto JA, Quatrano RS (2007) 14-3-3 Proteins are components of the transcription complex of the ATEM1 promoter in Arabidopsis. Planta 227:167–175
Fu H, Subramanian RR, Masters SC (2000) 14-3-3 proteins: structure, function, and regulation. Annu Rev Pharmacol 40:617–647
Gampala SS, Kim TW, He JX, Tang W, Deng Z, Bai MY, Shenheng G, Lalonde S, Sun Y, Gendron JM, Chen H, Shibagaki N, Ferl RJ, Ehrhardt D, Chong K, Burlingame AL, Wang ZY (2007) An essential role for 14-3-3 proteins in brassinosteroid signal transduction in Arabidopsis. Dev Cell 13:177–189
Hayashi M, Inoue SI, Takahashi K, Kinoshita T (2011) Immunohistochemical detection of blue light-induced phosphorylation of the plasma membrane H + -ATPase in stomatal guard cells. Plant Cell Physiol 52:1238–1248
Hayashi Y, Takahashi K, Inoue SI, Kinoshita T (2014) Abscisic acid suppresses hypocotyl elongation by dephosphorylating plasma membrane H + -ATPase in Arabidopsis thaliana. Plant Cell Physiol 55:845–853
He JX, Gendron JM, Yang Y, Li J, Wang ZY (2002) The GSK3-like kinase BIN2 phosphorylates and destabilizes BZR1, a positive regulator of the brassinosteroid signaling pathway in Arabidopsis. Proc Natl Acad Sci USA 99:10185–10190
Huber SC, MacKintosh C, Kaiser WM (2002) Metabolic enzymes as targets for 14-3-3 proteins. Plant Mol Biol 50:1053–1063
Ishida S, Fukazawa J, Yuasa T, Takahashi Y (2004) Involvement of 14-3-3 signaling protein binding in the functional regulation of the transcriptional activator repression of shoot growth by gibberellins. Plant Cell 16:2641–2651
Jarillo JA, Capel J, Leyva A, Martínez-Zapater JM, Salinas J (1994) Two related low-temperature-inducible genes of Arabidopsis encode proteins showing high homology to 14-3-3 proteins, a family of putative kinase regulators. Plant Mol Biol 25:693–704
Kagale S, Divi UK, Krochko JE, Keller WA, Krishna P (2007) Brassinosteroid confers tolerance in Arabidopsis thaliana and Brassica napus to a range of abiotic stresses. Planta 225:353–364
Kazan K (2015) Diverse roles of jasmonates and ethylene in abiotic stress tolerance. Trends Plant Sci 20:219–229
Keicher J, Jaspert N, Weckermann K, Möller C, Throm C, Kintzi A, Oecking C (2017) Arabidopsis 14-3-3 epsilon members contribute to polarity of PIN auxin carrier and auxin transport-related development. Elife 6:e24336
Kim TW, Guan S, Burlingame AL, Wang ZY (2011) The CDG1 kinase mediates brassinosteroid signal transduction from BRI1 receptor kinase to BSU1 phosphatase and GSK3-like kinase BIN2. Mol Cell 43:561–571
Kinoshita T, Caño-Delgado A, Seto H, Hiranuma S, Fujioka S, Yoshida S, Chory J (2005) Binding of brassinosteroids to the extracellular domain of plant receptor kinase BRI1. Nature 433:167
Koh S, Lee SC, Kim MK, Koh JH, Lee S, An G, Choe S, Kim SR (2007) T-DNA tagged knockout mutation of rice OsGSK1, an orthologue of Arabidopsis BIN2, with enhanced tolerance to various abiotic stresses. Plant Mol Biol 65:453–466
Krysan PJ, Young JC, Tax F, Sussman MR (1996) Identification of transferred DNA insertions within Arabidopsis genes involved in signal transduction and ion transport. Proc Natl Acad Sci USA 93:8145–8150
Li J, Nam KH (2002) Regulation of brassinosteroid signaling by a GSK3/SHAGGY-like kinase. Science 295:1299–1301
Lozano-Durán R, Robatzek S (2015) 14-3-3 proteins in plant–pathogen interactions. Mo Plant Microbe In 28:511–518
Medina J, Catalá R, Salinas J (2011) The CBFs: three Arabidopsis transcription factors to cold acclimate. Plant Sci 180:3–11
Mora-García S, Vert G, Yin Y, Caño-Delgado A, Cheong H, Chory J (2004) Nuclear protein phosphatases with Kelch-repeat domains modulate the response to brassinosteroids in Arabidopsis. Gene Dev 18:448–460
Oh MH, Wang X, Kota U, Goshe MB, Clouse SD, Huber SC (2009) Tyrosine phosphorylation of the BRI1 receptor kinase emerges as a component of brassinosteroid signaling in Arabidopsis. Proc Natl Acad Sci USA 106:658–663
Oh MH, Wang X, Clouse SD, Huber SC (2012) Deactivation of the Arabidopsis brassinosteroid insensitive 1 (BRI1) receptor kinase by autophosphorylation within the glycine-rich loop. Proc Natl Acad Sci USA 109:327–332
Ormancey M, Thuleau P, Mazars C, Cotelle V (2017) CDPKs and 14-3-3 proteins: emerging duo in signaling. Trends Plant Sci 22:263–272
Reiland S, Messerli G, Baerenfaller K, Gerrits B, Endler A, Grossmann J, Gruissem W, Baginsky S (2009) Large-scale Arabidopsis phosphoproteome profiling reveals novel chloroplast kinase substrates and phosphorylation networks. Plant Physiol 150:889–903
Rosenquist M, Alsterfjord M, Larsson C, Sommarin M (2001) Data mining the Arabidopsis genome reveals fifteen 14-3-3 genes. Expression is demonstrated for two out of five novel genes. Plant Physiol 127:142–149
Schoonheim PJ, Sinnige MP, Casaretto JA, Veiga H, Bunney TD, Quatrano RS, de Boer AH (2007) 14-3-3 adaptor proteins are intermediates in ABA signal transduction during barley seed germination. Plant J 49:289–301
Sirichandra C, Davanture M, Turk BE, Zivy M, Valot B, Leung J, Merlot S (2010) The Arabidopsis ABA-activated kinase OST1 phosphorylates the bZIP transcription factor ABF3 and creates a 14-3-3 binding site involved in its turnover. PLoS One 5:e13935
Sugiyama N, Nakagami H, Mochida K, Daudi A, Tomita M, Shirasu K, Ishihama Y (2008) Large-scale phosphorylation mapping reveals the extent of tyrosine phosphorylation in Arabidopsis. Mol Syst Biol 4:193
Tang W, Yuan M, Wang R, Yang Y, Wang C, Oses-Prieto JA, Kim TW, Zhou HW, Deng Z, Gampala SS, Gendron JM, Jonassen EM, Lillo C, DeLong A, Burlingame A, Sun Y, Wang ZY (2011) PP2A activates brassinosteroid-responsive gene expression and plant growth by dephosphorylating BZR1. Nat Cell Biol 13:124
van den Wijngaard PW, Sinnige MP, Roobeek I, Reumer A, Schoonheim PJ, Mol JN, Wang M, De Boer AH (2005) Abscisic acid and 14-3-3 proteins control K + channel activity in barley embryonic root. Plant J 41:43–55
van Kleeff PJM, Gao J, Mol S, Zwart N, Zhang H, Li KW, de Boer AH (2018) The Arabidopsis GORK K + -channel is phosphorylated by calcium-dependent protein kinase 21 (CPK21), which in turn is activated by 14-3-3 proteins. Plant Physiol Bioch 125:219–231
Wang X, Chory J (2006) Brassinosteroids regulate dissociation of BKI1, a negative regulator of BRI1 signaling, from the plasma membrane. Science 313:1118–1122
Wang ZY, Seto H, Fujioka S, Yoshida S, Chory J (2001) BRI1 is a critical component of a plasma-membrane receptor for plant steroids. Nature 410:380
Wang ZY, Nakano T, Gendron J, He J, Chen M, Vafeados D, Yang Y, Fujioka S, Yoshida S, Asami T, Chory J (2002) Nuclear-localized BZR1 mediates brassinosteroid-induced growth and feedback suppression of brassinosteroid biosynthesis. Dev Cell 2:505–513
Wang X, Li X, Meisenhelder J, Hunter T, Yoshida S, Asami T, Chory J (2005) Autoregulation and homodimerization are involved in the activation of the plant steroid receptor BRI1. Dev Cell 8:855–865
Wang X, Kota U, He K, Blackburn K, Li J, Goshe MB, Huber SC, Clouse SD (2008) Sequential transphosphorylation of the BRI1/BAK1 receptor kinase complex impacts early events in brassinosteroid signaling. Dev Cell 15:220–235
Wang H, Yang C, Zhang C, Wang N, Lu D, Wang J, Zhang S, Wang ZX, Ma H, Wang X (2011) Dual role of BKI1 and 14-3-3 s in brassinosteroid signaling to link receptor with transcription factors. Dev Cell 21:825–834
Wilson RS, Swatek KN, Thelen JJ (2016) Regulation of the regulators: post-translational modifications, subcellular, and spatiotemporal distribution of plant 14-3-3 proteins. Front Plant Sci 7:611
Yin Y, Wang ZY, Mora-Garcia S, Li J, Yoshida S, Asami T, Chory J (2002) BES1 accumulates in the nucleus in response to brassinosteroids to regulate gene expression and promote stem elongation. Cell 109:181–191
Acknowledgements
This work was supported in part by the Basic Science Research Program through the National Research Foundation of Korea (NRF), funded by the Ministry of Education (No. NRF-2017R1A2B4004620), and by Korea Institute of Planning and Evaluation for Technology in Food, Agriculture, Forestry and Fisheries (IPET) through the Golden Seed Project, Ministry of Agriculture, Food and Rural Affairs (MAFRA) (213006-05-3-SBC30).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Lee, J.H., Kwak, G., Lim, Y.P. et al. 14-3-3 proteins contribute to leaf and root development via brassinosteroid insensitive 1 in Arabidopsis thaliana. Genes Genom 42, 347–354 (2020). https://doi.org/10.1007/s13258-019-00909-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13258-019-00909-4