Abstract
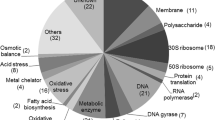
Gain-of-function mutations of Saccharomyces cerevisiae pleiotropic drug resistance (PDR) genes PDR1, PDR3, and YRR1 cause constitutive high expression of membrane-associated drug efflux pumps, resulting in the excretion of antifungal drugs. We previously identified a novel mutation of YRR1 that conferred salicylic acid (SA) resistance to S. cerevisiae BY4741 cells randomly mutagenized with ethyl methane sulfonate. Yeast cells carrying the yrr1–52 allele activated several drug efflux pumps and exhibited resistance to 4-nitroquinoline-N-oxide and cycloheximide. However, the activated genes and other factors that are directly involved in SA resistance remain unclear. Therefore, microarray analyses of total RNA extracted from yrr1–52 and wild-type cells during the logarithmic phase were performed. Twenty genes demonstrating >2-fold higher expression than the wild-type were selected. Among the upregulated genes, YOR1, SNQ2, AZR1, and FLR1 encode transport proteins previously associated with drug efflux pumps. YOR1 and SNQ2, which encode an ATP-binding cassette (ABC) transporter, were investigated as candidate genes involved in SA resistance. Spot assay results showed no changes in SA susceptibility after disruption of YOR1 or SNQ2 on a background of YRR1 and yrr1–52, indicating that Yor1p and Snq2p are not involved in SA resistance.

Similar content being viewed by others
References
Alarco AM, Balan I, Talibi D, Mainville N, Raymond M. AP1-mediated multidrug resistance in Saccharomyces cerevisiae requires FLR1 encoding a transporter of the major facilitator superfamily. Jour Biol Chem. 1997;272:19304–13.
Amberg DC, Burke DJ, Strathern JN. Methods in yeast genetics: a cold spring harbor laboratory course manual. New York: Cold Spring Harbor Laboratory Press; 2005.
Balzi E, Chen W, Ulaszewski S, Capieaux E, Goffeau A. The multidrug resistance gene PDR1 from Saccharomyces cerevisiae. Jour Biol Chem. 1987;262:16871–9.
Cannon RD, Lamping E, Holmes AR, Niimi K, Baret PV, Keniya MV, et al. Efflux-mediated antifungal drug resistance. Clin Microbiol Rev. 2009;22:291–321.
Cui Z, Shiraki T, Hirata D, Miyakawa T. Yeast gene YRR1, which is required for resistance to 4-nitroquinoline N-oxide, mediates transcriptional activation of the multidrug resistance transporter gene SNQ2. Mol Microbiol. 1998;29:1307–15.
Katzmann DJ, Hallstrom TC, Voet M, Wysock W, Golin J, Volckaert G, et al. Expression of an ATP-binding cassette transporter-encoding gene (YOR1) is required for oligomycin resistance in Saccharomyces cerevisiae. Mol Cellular Biol. 1995;15:6875–83.
Kodo N, Matsuda T, Doi S, Munakata H. Salicylic acid resistance is conferred by a novel YRR1 mutation in Saccharomyces cerevisiae. Biochem Biophys Res Commun. 2013;434:42–7.
Kohlhaw GB. Leucine biosynthesis in fungi: entering metabolism through the back door. Microbiol Mol Biol Rev. 2003;67:1–15.
Le Crom S, Devaux F, Marc P, Zhang X, Moye-Rowley WS, Jacq C. New insights into the pleiotropic drug resistance network from genome-wide characterization of the YRR1 transcription factor regulation system. Mol Cellular Biol. 2002;22:2642–9.
MacPherson S, Larochelle M, Turcotte B. A fungal family of transcriptional regulators: the zinc cluster proteins. Microbiol Mol Biol Rev. 2006;70:583–604.
Moye-Rowley WS. Transcriptional control of multidrug resistance in the yeast saccharomyces. Progress in Nucleic Acid Research and Molecular Biology. 2003;73:251–79.
Prasad R, Goffeau A. Yeast ATP-binding cassette transporters conferring multidrug resistance. Annu Rev Microbiol. 2012;66:39–63.
Sambrook J, Fritsch EF, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. New York: Cold Spring Harbor Laboratory Press; 1989.
Servos J, Haase E, Brendel M. Gene SNQ2 of Saccharomyces cerevisiae, which confers resistance to 4-nitroquinoline-N-oxide and other chemicals, encodes a 169 kDa protein homologous to ATP-dependent permeases. Mol Gen Genet. 1993;236:214–8.
Subik J, Ulaszewski S, Goffeau A. Genetic mapping of nuclear mucidin resistance mutations in Saccharomyces cerevisiae. A new pdr locus on chromosome II. Curr Genet. 1986;10:665–70.
Tenreiro S, Rosa PC, Viegas CA, Sa-Correia I. Expression of the AZR1 gene (ORF YGR224w), encoding a plasma membrane transporter of the major facilitator superfamily, is required for adaptation to acetic acid and resistance to azoles in Saccharomyces cerevisiae. Yeast. 2000;16:1469–81.
Acknowledgments
Naohiko Kodo would like to express the deepest appreciation to Prof. Yasuhiko Mukai for his support and encouragement.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kodo, N., Sakata, S. & Matsuda, T. Upregulated pleiotropic drug resistance genes in Saccharomyces cerevisiae yrr1–52 . Nucleus 58, 231–234 (2015). https://doi.org/10.1007/s13237-016-0156-5
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13237-016-0156-5