Abstract
Zika fever is an emerging mosquito-borne disease. While it often causes no or only mild symptoms that are similar to dengue fever, Zika virus can spread from a pregnant woman to her baby and cause severe birth defects. There is no specific treatment or vaccine, but the disease can be mitigated by using several control strategies, generally focusing on the reduction in mosquitoes or mosquito bites. In this paper, we model Zika virus transmission and incorporate a game-theoretical approach to study a repeated population game of DEET usage to prevent insect bites. We show that the optimal use effectively leads to disease elimination. This result is robust and not significantly dependent on the cost of the insect repellents.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Zika virus (ZIKV) is an emerging mosquito-borne flavivirus [34]. ZIKV is transmitted to humans through the bites of infected Aedes mosquitoes, including A. aegypti and A. albopictus [2]. Recent studies show that ZIKV can be transmitted between humans via sexual contact or by blood transfusions [32].
ZIKV infection is predominantly a mild or asymptomatic dengue-like disease [29]. ZIKV infection can lead to Guillain–Barré Syndrome, microcephaly and other severe birth defects [37].
There is currently no vaccine, specific treatment, or fast diagnostic test is available to treat, prevent, or diagnose ZIKV infection [33]. ZIKV mitigation is closely associated with the reduction in the presence and abundance of A. aegypti [9]. On the individual level, the best way to control the spread of ZIKV is to reduce sexual transmissions and avoid mosquito bites [62]. This can be achieved by avoiding unprotected sex [14, 59], using mosquito repellents [36], bed nets [57], window screens [42], and air conditioning [62]. The climate changes may be behind the recent rise of ZIKV and other Aedes-borne infections [64,65,66].
ZIKV received considerable attention from mathematical modelers; see for example [2, 3, 11, 17, 26, 43, 45, 47, 52, 61, 71, 73, 74].
Starting with [10], game theory has been increasingly applied to help with the modeling of the prevention of diseases such as African trypanosomiases [24], chikungunya [40], Ebola [13], Hepatitis C [67], meningitis [50], monkeypox [8], toxoplasmosis [70] and others; see [18, 75] for recent reviews.
In this paper, we build on the work of [12, 72]. We show their compartmental ODE model in Sect. 2. The main contribution of this paper is the use game theory to model ZIKV prevention by a voluntary use of insect repellents. The game-theoretical framework is introduced in Sect. 2.3 where we define a population game played by susceptible individuals who repeatedly decide whether to use DEET or not. Our main results, including the game theory analysis, are presented in Sect. 4. We give exact formulas for the minimal DEET coverage needed for ZIKV elimination. We also give numerical values for the Nash equilibria, i.e., the solution of the game and the optimal voluntary use of DEET to prevent the spread of ZIKV through vector bites. We show that the Nash equilibrium is high enough and thus the voluntary use of DEET can significantly contribute to ZIKV elimination.
2 Model
2.1 Compartmental Model
We adapt an SIR-SI compartmental ODE model of ZIKV dynamics originally introduced in [12] and extended in [72].
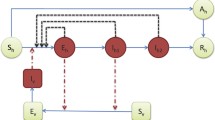
The total human population at time t, \(N_h(t)\), is divided into susceptible, \(S_h(t)\), infectious, \(I_h(t)\), and recovered, \(R_h(t)\). The mosquito population, \(N_v(t)\), is divided into susceptible, \(S_v(t)\), and infectious, \(I_v(t)\).
The basic model, without any control measures, is as follows. For simplicity, we assume no vertical transmission and thus all humans are recruited, at rate \(\varLambda _h\) as susceptible. The susceptible individuals can be infected in one of the following ways: (1) directly through contact with an infected individual which happens at rate \(\beta _{hh}\), or (2) through a bite by an infected mosquito, the effective mosquito-to-human transmission rate will be denoted \(\beta _{vh}\). The infectious individuals naturally recover at a rate of \(\gamma \). Recovered individuals are assumed to have acquired a permanent immunity. We assume no disease-induced mortality and denote the natural death rate by \(\mu _h\).
The mosquito recruitment rate is \(\varLambda _v\). We again assume that there is no vertical transmission and all mosquitoes are born susceptible. The mosquito natural death rate is \(\mu _v\). Mosquitoes are infected via contact with infected humans at a rate of \(\beta _{hv}\).
There are four control measures:
-
1.
The bite control, \(c_b\), such as using the insect repellent. This control limits the human-to-mosquito and mosquito-to-human contact rate and causes the effective transmission rates to be \(\widetilde{\beta }_{hv}(c_b)= (1-c_b)\beta _{hv}\) and \(\widetilde{\beta }_{vh}(c_b)=(1-c_b)\beta _{vh}\), respectively. Here, we use a linear dependence since, at least in the first approximation, being unprotected for double the time doubles the probability of transmission. We note, however, that other functions may be more appropriate.
-
2.
The human-to-human contact control, \(c_c\), such as the use of condoms. This control causes the effective human-to-human transmission rate to be \(\widetilde{\beta }_{hh}(c_c)= (1-c_c)\beta _{hh}\). As above, we use a linear dependence since, at least in the first approximation, being unprotected in twice as many interactions doubles the probability of transmission. We note, however, that other functions may be more appropriate.
-
3.
The treatment control, \(c_t\), which causes recovery to progress at a faster rate \(\widetilde{\gamma }(c_t)= \gamma + c_t\gamma _{h,t}\).
-
4.
The insecticide control, \(c_i\), which increases the mosquito death rate to \(\widetilde{\mu }_v(c_i)= \mu _v+c_i\mu _{v,i}\).
For simplicity, all control measures are assumed to be constants and in the interval [0, 1].
The dynamics of the compartmental ODE model are summarized in Fig. 1. See Table 1 for a summary of the notation and values of the parameters.
The model in Fig. 1, which is a special case of a system considered in [76], yields the following differential equations.
2.2 Model Calibration
We adopt most of the parameter values from [72] who followed [25] who fit the ZIKV transmission model to data from Brazil.
The recruitment rate, \(\varLambda _h\), in Brazil is 13.92 per year per 1000 people [77]. The life expectancy, \(\mu _h^{-1}\), in Brazil is 74 years [23]. It takes on average 9.9 days for the infected individual to have no detectable virus in blood [46], the infectiousness of ZIKV infection ends 1.5–2 days before the virus becomes undetectable [30]. Thus, an individual stays infectious for \(\gamma ^{-1} = 7.9\) days [25]. The mean duration of the acute ZIKV phase is 5 days [33], which gives \(\gamma _{h,t}=\frac{1}{5}\).
The mosquito recruitment rate, \(\varLambda _v\), is about 3000 a day [4]. The mosquito life expectancy, \(\mu _v^{-1}\), is 11 days [60]. This is in line with the life expectancy for mosquitoes in Rio de Janeiro, Brazil [49], and close to the average of 2–3 weeks considered in biological studies [58].
The time between a mosquito being infected and it infecting a human, \(\beta _{vh}^{-1}\), is 11.3 days [25], while the time between a human infection and a mosquito taking an infectious blood meal, \(\beta _{hv}^{-1}\), is and 8.6 days, respectively [30]. The human-to-human transmission rate, \(\beta _{hh}\), is \(\frac{1}{20}\) [33].
The insecticide related death rate, \(\mu _{v,i}\) is 1, about 10 times the natural mosquito mortality rate, in line with [56].
We estimate the bite control, \(c_b\) as follows. The population of Brazil is about 209 mil people [77]. In 2015, the revenue from mosquito repellent sales in Brazil was $55.7 mil [16]. The revenue for 2016 was expected to rise 120% [16], i.e., to $122.54 mil. The most frequent DEET repellents cost about $4.2 [53], i.e., about 29.17 mil bottles were sold in 2016. One bottle of DEET contains about 400 sprays [28, 54], i.e., lasts about 200 days since, on average, DEET is applied twice a day [53]. At the same time, three applications of DEET per day seem to provide complete protection. We thus get \(c_b = (209\times 10^6)^{-1} \cdot (29.17\times 10^6) \cdot \left( \frac{200}{365}\right) \cdot \left( \frac{2}{3}\right) = 0.051\).
We were not able to obtain values of the controls \(c_i, c_c, c_t\) from the literature, but we calibrated the model as follows. There were 215319 ZIKV infection cases in 2016 [48, 55], i.e., the incidence rate was 103 cases in \(10^5\) people. Setting \(c_c=0.05\), \(c_t=0.05\) and \(c_i = 0.005\) yielded an incidence rate of 106 cases per \(10^5\) people and so we adopted those values. We note that there are many other combinations of values that yield the similar incidence rate. However, the main results are quite robust to a particular choice of this combination since similar incidence rates yield similar risks of infections.
Most ZIKV infections are asymptomatic and do not need any extra costs. The cost of a symptomatic ZIKV infection in the US is around $6355 dollars [45]. The GDP per capita for Brazil is $15600 and for the United States is $59500 [23]. Therefore, the cost of ZIKV infection in Brazil is estimated as $1588.
The cost of mosquito bite prevention by using DEET is estimated as follows. The cost of the most frequently sold DEET is $4.2 per bottle [53]. One bottle of DEET contains 400 sprays and at three sprays a day, the cost is about $0.0315 per day.
2.3 Game-Theoretical Framework of Voluntary Bite Protection
Following [10], we add a game-theoretical component to the compartmental model above. We consider a repeated population game where the payoff to each individual is determined by their own strategy and the average strategy used by the population as a whole.
The players of the game are susceptible individuals who repeatedly choose whether to use DEET or not. We characterize the strategy by a single number \(c_b\) that specifies a proportion of the time the individual uses DEET. The solution of the game, called the Nash equilibrium, is the population-level value \(c_{b,\mathrm {NE}}\) at which no individual can increase their own payoff by deviating from the population strategy.
The payoff to an individual depends on the prevalence of ZIKV in the population, i.e., on the strategies other players are using. To solve for the Nash equilibrium value, we can assume that all players use the same strategy and only the strategy of the focal player may vary. Following [10], we assume that all individuals are provided with the same information such as prevalence of ZIKV in the population, the cost of treatment, and the cost of DEET. We will also assume that they use the information in the same and rational way to assess costs and risks [10].
The probability of getting infected when not using DEET at all while everybody else is using it at frequency \(c_b\) is
which corresponds to the rate moving from \(S_h\) to \(I_h\) (when not using any DEET protection) over the total rate of moving out of \(S_h\). The probability may (and will) depend on \(c_b\), because the formula contains \(I_v\) and \(I_h\) that may (and will) depend on \(c_b\). The average cost of not using DEET, \(C_N\), when everybody else uses it at frequency \(c_b\) is then given by
In order to determine the expected cost of using DEET, let \(C_{\mathrm {DEET}}\) denote the cost of actual use. Because one can still get infected from another human, the probability of infection is
So, the cost of using DEET, \(C_U\), when everybody else is using it at frequency \(c_b\) is given by
To get Nash equilibrium, we have to solve
for \(c_b\).
3 Analysis of the ODE System
There are two equilibria of the differential equations given in (1)–(5). The equilibria and the basic reproduction number were derived in [72]; here we summarize the main results.
3.1 Disease-free Equilibrium
The disease-free equilibrium \(E^0 = (S_H^0, I_H^0, R_H^0, S_V^0, I_V^0)\) is given by
The basic reproduction number, \(\mathcal {R}_0\), is given by
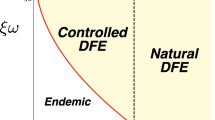
Since system (1)–(5) is a special case of a system considered in [76], Theorems 2.1.1 and 2.1.2 of [76] directly imply that, the disease-free equilibrium is globally asymptotically stable if \(\mathcal {R}_0\le 1\). It is unstable if \(\mathcal {R}_0>1\).
3.2 Endemic Equilibrium
The endemic equilibrium is \(E^*=(S_h^*, I_h^*, R_h^*, S_v^*, I_v^*)\) where \(I_h^*\) is a positive solution of
with
and
If \(\mathcal {R}_0>1\), the endemic equilibrium is unique [72] and locally asymptotically stable [76, Theorem 2.2.1].
4 Results
4.1 Minimal DEET Coverage Needed for ZIKV Elimination
In this section, we determine \(c_{b,\emptyset }\), the minimal coverage at which susceptible individuals should use DEET so that ZIKV is eliminated. It follows from (12) that \(\frac{\partial R_0}{\partial c_b}<0\), i.e., \(\mathcal {R}_0\) is decreasing in \(c_b\). Consequently, ZIKV will be eliminated at the smallest value of \(c_b\in [0,1]\) for which \(R_0\le 1\). Note that if \(\mathcal {R}_0|_{c_b=1}= \frac{1}{\mu _H+\widetilde{\gamma }(c_t)} \cdot \widetilde{\beta }_{hh}(c_c)>1\), then \(\mathcal {R}_0>1\) for all values of \(c_b\) and thus ZIKV will never be eliminated. On the other hand, if \(\mathcal {R}_0|_{c_b=0}<1\), i.e., \(\mathcal {R}_0<1\) even when nobody is using DEET, then ZIKV is not endemic. The only non-trivial critical value of \(c_b\) exists when \(\mathcal {R}_0|_{c_b=0}>1\) and \(\mathcal {R}_0|_{c_b=1}<1\). By (12) it is given as a solution of
Consequently,
4.2 Nash Equilibrium
In this section, we are interested in finding \(c_{b,\mathrm {NE}}\). We will consider the situation only under the endemic equilibrium (there is no need to use DEET in the disease-free equilibrium).
The expected cost of not using DEET (solid line) and the expected cost of using DEET as a function of population-level DEET coverage in the population. The parameters are as in Table 1. The solid line reaches 0 at \(c_{b,\emptyset }\), the level needed for ZIKV elimination. The lines intersect at the Nash equilibrium, \(c_{b,\mathrm {NE}}\). While \(c_{b,\mathrm {NE}}< c_{b,\emptyset }\), we can see that \(c_{b,\mathrm {NE}}\approx c_{b,\emptyset }\). In fact, in our model \(c_{b,\emptyset }-c_{b,\mathrm {NE}}<10^{-3}\) even when parameter values differ significantly from the values specified in Table 1
The graphical solution of \(C_N(c_b) = C_U(c_b)\) where \(C_N\) and \(C_U\) are given in (7) and (9) is illustrated in Fig. 2. The numerical solutions are shown in Fig. 3.
For the parameter values in Table 1, the resulting social optimum and Nash equilibrium values are \(c_{b,\emptyset }= 0.0886\) and \(c_{b,\mathrm {NE}}=0.0885\), respectively. The annual incidence rate when individuals use the optimal voluntary level of protection, \(c_{b,\mathrm {NE}}\), is 0.2 ZIKV cases per \(10^5\) people. We can thus see that the disease would be almost eliminated by optimal voluntary use of DEET.
We can also consider the annual incidence rate of 0.2 cases per \(10^5\) people to be the price of anarchy as if the social optimum, \(c_{b,\emptyset }\), was enforced, ZIKV would be eliminated, i.e., the incidence rate would be 0.
4.3 Sensitivity Analysis
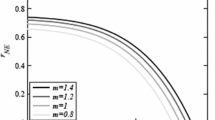
The dependence of \(c_{b,\mathrm {NE}}\) on parameters is shown in Fig. 3 and the sensitivity indices are presented in Table 2. The values of \(c_{b,\emptyset }\) track closely the values of \(c_{b,\mathrm {NE}}\) with only a minimal difference (less than \(10^{-3}\)) between the two.
The optimal voluntary level of protection, \(c_{b,\mathrm {NE}}\), is essentially constant in the human recruitment rate \(\varLambda _h\), the vector recruitment rate \(\lambda _v\), the human natural death rate \(\mu _h\), the cost of the prevention, \(C_{\mathrm {DEET}}\), and the cost of the disease, \(C_{\mathrm {Zika}}\).
Dependence of \(c_{b,\mathrm {NE}}\) on different parameter values. Unless varied, the parameter values are as specified in Table 1. When all parameters are as in Table 1, \(c_{b,\emptyset }= 0.0886\) and \(c_{b,\mathrm {NE}}= 0.0885\). \(c_{b,\mathrm {NE}}\) is constant in \(\varLambda _h, \varLambda _v\) and \(\mu _h\) and these figures are not shown
Also, \(c_{b,\mathrm {NE}}\) increases rapidly with the human-to-vector and vector-to-human transmission rates, \(\beta _{hv}\) and \(\beta _{vh}\) and it is also increasing in the human-to-human transmission rate \(\beta _{hh}\). It is rapidly decreasing in the recovery rate \(\gamma \) and the mosquito death rate \(\widetilde{\mu }_v(c_i)\). Correspondingly, \(c_{b,\mathrm {NE}}\) is also decreasing in the treatment control \(c_t\), the insecticide control \(c_i\) and the contact control \(c_c\).
5 Discussion
One of the interesting predictions of this model is that the optimal voluntary use of DEET does not depend in any significant way on the cost of the prevention. This finding is in sharp contrast to other similar game-theoretical models. For example, as shown in [1], the relatively low cost of a typhoid infection (compared to vaccination cost and effectiveness) is the reason why the vaccination coverage is quite low in rural Ghana. Similarly, even for vector-borne diseases such as Chagas disease, the cost of the insecticide-treated nets significantly affects the optimal usage levels and consequently the disease incidence rate [35].
Another interesting prediction is that the optimal voluntary use of DEET brings ZIKV very close to elimination levels. This agrees with predictions for other vector-borne diseases such as malaria [15], dengue [27], chikungunya [40] and visceral leishmaniasis [31] or diseases like Ebola [13]. In all these cases, cost of disease prevention is low relative to the cost of the disease. This is different in vaccination games for diseases like polio [10, 19] and Hepatitis B vaccination [21, 68] where either the real or the perceived vaccination costs are relatively large.
The high cost of ZIKV infection can explain why the ZIKV outbreaks, while serious, are still relatively limited. It has been demonstrated that individuals act in a way that maximizes their self-interests, rather than the interests of the entire group [51]. Vaccination in particular and disease prevention in general is prone to free-riding [38]. The “free-riders” avoid the costs associated with the prevention while benefiting from actions taken by others [18, 69, 75]. However, the risk of severe negative ZIKV complications such as Guillain-Barré Syndrome, microcephaly, and other birth defects means that the cost of ZIKV infection is high and thus the individuals want to protect themselves (and their unborn babies) almost regardless of the cost of the protection.
Our model predicted the optimal voluntary use of the repellents at about 8.8%. On one hand, this number seems quite low. Indeed, in Northern Brazil where the ZIKV outbreak originated, the repellent use during 2016 was 83% among highly educated pregnant women and 56% among pregnant women overall [53]. However, in the very same region, only less than 21% of women of childbearing age (15–49 years) report having used mosquito repellent or insecticides frequently during 2015–2016 [63]. The economic factor was the main justification for the non-use of repellents both in urban and rural areas [53].
6 Conclusions
Mathematical modeling is a useful tool that helps our understandings of how interactions between the population, individuals, and the environment can change the course of a disease [22]. In this paper, we adopted an ODE model of ZIKV transmission dynamics that originated in [12]. We applied a game-theoretical approach, developed by Bauch and Earn [10], and determined the optimal voluntary use of DEET to prevent the spread of ZIKV through vector bites. We also gave formulas for a desired level of DEET usage that leads to ZIKV elimination. Similarly to other recently studied vector-borne diseases, such as dengue [27], malaria [15], visceral leishmaniasis [31] and Chagas [35], we saw that the optimal voluntary use of DEET can significantly contribute to ZIKV elimination. This differs from the predictions for non-vector-borne diseases such as polio [19] and hepatitis B [21] where the optimal voluntary use levels and the levels required for the herd immunity were significantly different.
There are several ways in which our model can be extended. From the game-theoretical perspective, one can apply a multi-agent-simulation (MAS) methodology [5, 39, 44]. This approach may be fruitful for two reasons: (1) the DEET application needs to be repeated as with the influenza [6], typhoid [1] or hepatitis [21, 68] vaccinations, (2) there are multiple ways in which one can get infected and the simulations may provide a more realistic and flexible approach for multiple protective actions. This would also allow the incorporation of game-theoretical component into models like [2, 3, 33] that distinguish between females and males.
There are four control measures that help ZIKV elimination. An individual generally cannot influence the level of hospital care or any large-scale insecticide spraying to substantially increase mosquito death rates; therefore the control measures related to these are not suitable for game-theoretical analysis. At the same time, the reduction in human-to-human transmission through the use of condoms is something an individual can influence directly. However, even this control is still not an individual’s own decision. Unless both individuals agree to use (or not to use) condom, there is no interaction. The proper analysis of the game involving condom use would thus have to take into account a population heterogeneity and contact network structure rather than just simple averages of players’ condom usage.
The analysis of game-theoretical models that deal with two or more prevention measures simultaneously seems to be much harder. So far only specific models for COVID-19 with vaccination or social distancing [20] and cholera with vaccination or clean water usage [41] were considered. It would be of a great interest to develop a general framework.
Data Availability
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.
References
Acosta-Alonzo CB, Erovenko IV, Lancaster A, Oh H, Rychtář J, Taylor D (2020) High endemic levels of typhoid fever in rural areas of Ghana may stem from optimal voluntary vaccination behavior. Proc R Soc A 476:20200354
Agusto FB, Bewick S, Fagan W (2017) Mathematical model of Zika virus with vertical transmission. Infect Dis Model 2(2):244–267
Agusto FB, Bewick S, Fagan WF (2017) Mathematical model for Zika virus dynamics with sexual transmission route. Ecol Complex 29:61–81
Andraud M, Hens N, Marais C, Beutels P (2012) Dynamic epidemiological models for dengue transmission: a systematic review of structural approaches. PLoS ONE 7(11):e49085
Arefin MR, Kabir KA, Tanimoto J (2020) A mean-field vaccination game scheme to analyze the effect of a single vaccination strategy on a two-strain epidemic spreading. J Stat Mech Theory Exp 2020(3):033501
Arefin MR, Masaki T, Kabir KA, Tanimoto J (2019) Interplay between cost and effectiveness in influenza vaccine uptake: a vaccination game approach. Proc R Soc A 475(2232):20190608
Arriola L, Hyman JM (2009) Sensitivity analysis for uncertainty quantification in mathematical models. In: Mathematical and statistical estimation approaches in epidemiology. Springer, pp 195–247
Bankuru SV, Kossol S, Hou W, Mahmoudi P, Rychtář J, Taylor D (2020) A game-theoretic model of Monkeypox to assess vaccination strategies. PeerJ 8:e9272
Basso C, da Rosa EG, Lairihoy R, Caffera RM, Roche I, González C, da Rosa R, Gularte A, Alfonso-Sierra E, Petzold M (2017) Scaling up of an innovative intervention to reduce risk of dengue, chikungunya, and zika transmission in Uruguay in the framework of an intersectoral approach with and without community participation. Am J Trop Med Hyg 97(5):1428–1436
Bauch CT, Earn DJ (2004) Vaccination and the theory of games. Proc Natl Acad Sci 101(36):13391–13394
Bonyah E, Khan MA, Okosun KO, Gómez-Aguilar J (2019) On the co-infection of dengue fever and Zika virus. Optim Control Appl Methods 40(3):394–421
Bonyah E, Okosun KO (2016) Mathematical modeling of Zika virus. Asian Pacific J Trop Dis 6(9):673–679
Brettin A, Rossi-Goldthorpe R, Weishaar K, Erovenko IV (2018) Ebola could be eradicated through voluntary vaccination. R Soc Open Sci 5(1):171591
Brooks JT, Friedman A, Kachur RE, LaFlam M, Peters PJ, Jamieson DJ (2016) Update: interim guidance for prevention of sexual transmission of Zika virus—United States, July 2016. Morb Mortal Wkly Report 65(29):745–747
Broom M, Rychtář J, Spears-Gill T (2016) The game-theoretical model of using insecticide-treated bed-nets to fight malaria. Appl Math 7(09):852–860
Bruno L (2016) Mosquito repellant sales boom in Brazil amid Zika scare. https://www.reuters.com/article/health-zika-repellant/mosquito-repellant-sales-boom-in-brazil-amid-zika-scare-idUSL8N15I52T. Accessed 10 Nov 2020
Carran S, Ferrari M, Reluga T (2018) Unintended consequences and the paradox of control: management of emerging pathogens with age-specific virulence. PLoS Negl Trop Dis 12(4):e0005997
Chang SL, Piraveenan M, Pattison P, Prokopenko M (2020) Game theoretic modelling of infectious disease dynamics and intervention methods: a review. J Biol Dyn 14(1):57–89
Cheng E, Gambhirrao N, Patel R, Zhowandai A, Rychtář J, Taylor D (2020) A game-theoretical analysis of Poliomyelitis vaccination. J Theor Biol 499:110298
Choi W, Shim E (2020) Optimal strategies for vaccination and social distancing in a game-theoretic epidemiologic model. J Theor Biol 505:110422
Chouhan A, Maiwand S, Ngo M, Putalapattu V, Rychtář J, Taylor D (2020) Game-theoretical model of retroactive hepatitis B vaccination in China. Bull Math Biol 82:80
Chubb MC, Jacobsen KH (2010) Mathematical modeling and the epidemiological research process. Eur J Epidemiol 25(1):13–19
CIA (2020) The world factbook—birth rate. https://www.cia.gov/library/publications/the-world-factbook/fields/345.html. Accessed 13 Apr 2020
Crawford K, Lancaster A, Oh H, Rychtář J (2015) A voluntary use of insecticide-treated cattle can eliminate African sleeping sickness. Lett Biomath 2(1):91–101
Dantas E, Tosin M, Cunha A Jr (2018) Calibration of a SEIR-SEI epidemic model to describe the Zika virus outbreak in Brazil. Appl Math Comput 338:249–259
Ding C, Tao N, Zhu Y (2016) A mathematical model of Zika virus and its optimal control. In: 2016 35th Chinese control conference (CCC). IEEE, pp 2642–2645
Dorsett C, Oh H, Paulemond ML, Rychtář J (2016) Optimal repellent usage to combat dengue fever. Bull Math Biol 78(5):916–922
EPA (2020) US Environmental Protection Agency: find the repellent that is right for you. https://www.epa.gov/insect-repellents/find-repellent-right-you. Accessed 10 Nov 2020
Fauci AS, Morens DM (2016) Zika virus in the Americas-yet another arbovirus threat. N Engl J Med 374(7):601–604
Ferguson NM, Cucunubá ZM, Dorigatti I, Nedjati-Gilani GL, Donnelly CA, Basáñez M-G, Nouvellet P, Lessler J (2016) Countering the Zika epidemic in Latin America. Science 353(6297):353–354
Fortunato AK, Glasser CP, Watson JA, Lu Y, Rychtář J, Taylor D (2021) Mathematical modelling of the use of insecticide-treated nets for elimination of visceral leishmaniasis in Bihar, India. R Soc Open Sci 8(6):201960
Foy BD, Kobylinski KC, Foy JLC, Blitvich BJ, da Rosa AT, Haddow AD, Lanciotti RS, Tesh RB (2011) Probable non-vector-borne transmission of Zika virus, Colorado, USA. Emerg Infect Dis 17(5):880
Gao D, Lou Y, He D, Porco TC, Kuang Y, Chowell G, Ruan S (2016) Prevention and control of Zika as a mosquito-borne and sexually transmitted disease: a mathematical modeling analysis. Sci Rep 6:28070
Hamel R, Dejarnac O, Wichit S, Ekchariyawat P, Neyret A, Luplertlop N, Perera-Lecoin M, Surasombatpattana P, Talignani L, Thomas F (2015) Biology of Zika virus infection in human skin cells. J Virol 89(17):8880–8896
Han CY, Issa H, Rychtář J, Taylor D, Umana N (2020) A voluntary use of insecticide treated nets can stop the vector transmission of Chagas disease. PLOS Negl Trop Dis 14(11):e0008833
Hennessey M, Fischer M, Staples JE (2016) Zika virus spreads to new areas—region of the Americas, May 2015–January 2016. Am J Transplant 16(3):1031–1034
Honein MA, Dawson AL, Petersen EE, Jones AM, Lee EH, Yazdy MM, Ahmad N, Macdonald J, Evert N, Bingham A et al (2017) Birth defects among fetuses and infants of US women with evidence of possible Zika virus infection during pregnancy. JAMA 317(1):59–68
Ibuka Y, Li M, Vietri J, Chapman GB, Galvani AP (2014) Free-riding behavior in vaccination decisions: an experimental study. PLoS ONE 9(1):e87164
Kabir KA, Jusup M, Tanimoto J (2019) Behavioral incentives in a vaccination-dilemma setting with optional treatment. Phys Rev E 100(6):062402
Klein SRM, Foster AO, Feagins DA, Rowell JT, Erovenko IV (2020) Optimal voluntary and mandatory insect repellent usage and emigration strategies to control the chikungunya outbreak on Reunion Island. PeerJ 8:e10151
Kobe J, Pritchard N, Short Z, Erovenko IV, Rychtář J, Rowell JT (2018) A game-theoretic model of cholera with optimal personal protection strategies. Bull Math Biol 80(10):2580–2599
Kroeger A, Lenhart A, Ochoa M, Villegas E, Levy M, Alexander N, McCall P (2006) Effective control of dengue vectors with curtains and water container covers treated with insecticide in Mexico and Venezuela: cluster randomised trials. BMJ 332(7552):1247–1252
Kucharski AJ, Funk S, Eggo RM, Mallet H-P, Edmunds WJ, Nilles EJ (2016) Transmission dynamics of Zika virus in island populations: a modelling analysis of the 2013–14 French Polynesia outbreak. PLoS Negl Trop Dis 10(5):e0004726
Kuga K, Tanimoto J, Jusup M (2019) To vaccinate or not to vaccinate: a comprehensive study of vaccination-subsidizing policies with multi-agent simulations and mean-field modeling. J Theor Biol 469:107–126
Lee BY, Alfaro-Murillo JA, Parpia AS, Asti L, Wedlock PT, Hotez PJ, Galvani AP (2017) The potential economic burden of Zika in the continental United States. PLoS Negl Trop Dis 11(4):e0005531
Lessler J, Ott CT, Carcelen AC, Konikoff JM, Williamson J, Bi Q, Kucirka LM, Cummings DA, Reich NG, Chaisson LH (2016) Times to key events in Zika virus infection and implications for blood donation: a systematic review. Bull World Health Organ 94(11):841
Li Y, Liu X (2020) Modeling and control of mosquito-borne diseases with Wolbachia and insecticides. Theor Popul Biol 132:82–91
Lowe R, Barcellos C, Brasil P, Cruz OG, Honório NA, Kuper H, Carvalho MS (2018) The zika virus epidemic in Brazil: from discovery to future implications. Int J Environ Res Public Health 15(1):96
Maciel-De-Freitas R, Codeco CT, Lourenco-De-Oliveira R (2007) Daily survival rates and dispersal of Aedes aegypti females in Rio de Janeiro, Brazil. Am J Trop Med Hyg 76(4):659–665
Martinez A, Machado J, Sanchez E, Erovenko I (2019) Optimal vaccination strategies to reduce endemic levels of meningitis in Africa. Preprint
Maskin E (1999) Nash equilibrium and welfare optimality. Rev Econ Stud 66(1):23–38
Maxian O, Neufeld A, Talis EJ, Childs LM, Blackwood JC (2017) Zika virus dynamics: when does sexual transmission matter? Epidemics 21:48–55
Melo VD, Silva JS, La Corte R (2019) Use of mosquito repellents to protect against Zika virus infection among pregnant women in Brazil. Public Health 171:89–96
Miller P (2004) Avoiding the bite: update on DEET: safe and effective against West Nile virus when properly used. Can Pharm J/Rev Pharm Can 137(5):44–47
Ministério da Saúde Brasília (2017) Secretaria de vigilância em saúde-ministério da saúde monitoramento dos casos de dengue, febre de chikungunya e febre pelo vírus zika até a semana epidemiológica 52, 2016. Bol Epidemiol 48(3):1–11
Momoh AA, Fügenschuh A (2018) Optimal control of intervention strategies and cost effectiveness analysis for a Zika virus model. Oper Res Health Care 18:99–111
Nahlen BL, Clark JP, Alnwick D (2003) Insecticide-treated bed nets. Am J Trop Med Hyg 68(4\(_{-}\)suppl):1–2
Nelson MJ (1986) Aedes aegypti: biology and ecology. Pan American Health Organization, Washington
Oduyebo T, Petersen EE, Rasmussen SA, Mead PS, Meaney-Delman D, Renquist CM, Ellington SR, Fischer M, Staples JE, Powers AM (2016) Update: interim guidelines for health care providers caring for pregnant women and women of reproductive age with possible Zika virus exposure—United States, 2016. Morb Mortal wkly Rep 65(5):122–127
Otero M, Solari HG, Schweigmann N (2006) A stochastic population dynamics model for Aedes aegypti: formulation and application to a city with temperate climate. Bull Math Biol 68(8):1945–1974
Padmanabhan P, Seshaiyer P, Castillo-Chavez C (2017) Mathematical modeling, analysis and simulation of the spread of Zika with influence of sexual transmission and preventive measures. Lett Biomath 4(1):148–166
Petersen LR, Jamieson DJ, Powers AM, Honein MA (2016) Zika virus. N Engl J Med 374(16):1552–1563
Quintana-Domeque C, Carvalho JR, de Oliveira VH (2018) Zika virus incidence, preventive and reproductive behaviors: correlates from new survey data. Econ Hum Biol 30:14–23
Robert MA, Christofferson RC, Silva NJ, Vasquez C, Mores CN, Wearing HJ (2016) Modeling mosquito-borne disease spread in US urbanized areas: the case of dengue in Miami. PLoS ONE 11(8):e0161365
Robert MA, Christofferson RC, Weber PD, Wearing HJ (2019) Temperature impacts on dengue emergence in the United States: investigating the role of seasonality and climate change. Epidemics 28:100344
Robert MA, Stewart-Ibarra AM, Estallo EL (2020) Climate change and viral emergence: evidence from Aedes-borne arboviruses. Curr Opin Virol 40:41–47
Scheckelhoff K, Ejaz A, Erovenko IV (2019) A game-theoretic model of optimal clean equipment usage to prevent hepatitis C among injecting drug users. Preprint
Scheckelhoff K, Ejaz A, Erovenko IV, Rychtář J, Taylor D (2021) Optimal voluntary vaccination of adults and adolescents can help eradicate hepatitis B in China. Games 12(4):82
Serpell L, Green J (2006) Parental decision-making in childhood vaccination. Vaccine 24(19):4041–4046
Sykes D, Rychtář J (2015) A game-theoretic approach to valuating toxoplasmosis vaccination strategies. Theor Popul Biol 105:33–38
Tang B, Xiao Y, Wu J (2016) Implication of vaccination against dengue for Zika outbreak. Sci Rep 6:35623
Taylor D (2021) Mathematical model of Zika virus transmission and control measures. N C J Math Stat 7:1–12
Turelli M, Barton NH (2017) Deploying dengue-suppressing Wolbachia: robust models predict slow but effective spatial spread in Aedes aegypti. Theor Popul Biol 115:45–60
Valega-Mackenzie W, Ríos-Soto KR (2018) Can vaccination save a Zika virus epidemic? Bull Math Biol 80(3):598–625
Verelst F, Willem L, Beutels P (2016) Behavioural change models for infectious disease transmission: a systematic review (2010–2015). J R Soc Interface 13(125):20160820
Wei H-M, Li X-Z, Martcheva M (2008) An epidemic model of a vector-borne disease with direct transmission and time delay. J Math Anal Appl 342(2):895–908
World Bank (2020) Life expectancy at birth. https://data.worldbank.org/indicator/SP.DYN.LE00.IN?cid=GPD_10. Accessed 13 Apr 2020
Acknowledgements
The authors would like to thank the anonymous reviewers and the guest editors for their constructive feedback and suggestions that helped to improve this manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
In the memory of J.V.
Rights and permissions
About this article
Cite this article
Angina, J., Bachhu, A., Talati, E. et al. Game-Theoretical Model of the Voluntary Use of Insect Repellents to Prevent Zika Fever. Dyn Games Appl 12, 133–146 (2022). https://doi.org/10.1007/s13235-021-00418-8
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13235-021-00418-8