Abstract
In this work, pH/GSH-responsive amphiphilic polymeric prodrug (EDA-GLA/CE/2-FPBA) was successfully prepared and could self-assembled into micelles in an aqueous solution. The EDA-GLA/CE/2-FPBA micelles possessed high stability in physiological condition and were pH and GSH sensitive due to the reversible borate ester bonds and disulfide bonds within the prodrug polymer. The structures of the prodrug polymers were characterized by NMR, FTIR, UV–vis spectroscopy. Transmission electron microscopy and dynamic light scattering measurement indicated that the resulting micelles have desirable size distribution and regular spherical shape. Free active Celastrol can be released under low pH and high GSH environment; In vitro cellular uptake and growth inhibition assays suggested that the blank polymer micelles showed good biocompatibility. EDA-GLA/CE/2-FPBA micelles were more efficiently internalized by monolayer tumor cells and demonstrated superior tumor targeting effects as compared to free Celastrol control. These results demonstrated that the novel prodrug self-assembled dual-responsive nano-delivery platform was able to improve the bioavailability and tumor targeting activity of Celastrol, which provides a basis for further clinical applications of Celastrol and its derivatives.
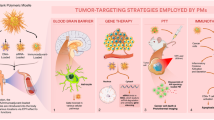
Graphical Abstract
Amphiphilic polymeric prodrug (EDA-GLA/CE/2-FPBA) containing gelatin, lipoic acid, ethylenediamine (EDA), 2-formylphenylboric acid (2-FPBA) was developed, which can self-assembled into micelles in an aqueous solution. Borate ester bond and sulfhydryl groups in the micelles endow the micelles with the ability to respond to high concentration of GSH.









Similar content being viewed by others
References
X. Wang, L. Yang, Z. Chen, D.M. Shin, Application of nanotechnology in cancer therapy and imaging. CA Cancer J. Clin. 58(2), 97–110 (2010)
K.M. Morrissey, T.M. Yuraszeck, C.-C. Li, Y. Zhang, S. Kasichayanula, Immunotherapy and novel combinations in oncology: current landscape, challenges, and opportunities. Clin. Transl. Sci. 9(2), 89–104 (2016)
K.D. Miller, L. Nogueira, T. Devasia, A.B. Mariotto, K.R. Yabroff, A. Jemal, J. Kramer et al., Cancer treatment and survivorship statistics, 2022. CA Cancer J. Clin. 72(5), 409–436 (2022)
T.J. Royce, M.M. Qureshi, M.T. Truong, Radiotherapy utilization and fractionation patterns during the first course of cancer treatment in the United States from 2004 to 2014. J. Am. Coll. Radiol. 15(11), 1558–1564 (2018)
J.M. Brown, A.J. Giaccia, The unique physiology of solid tumors: opportunities (and problems) for cancer therapy. Can. Res. 58(7), 1408–1416 (1998)
M. Das, C. Mohanty, Sanjeeb K Sahoo, Ligand-based targeted therapy for cancer tissue. Expert Opin. Drug Deliv. 6(3), 285–304 (2009)
P. Mi, N. Nishiyama, Polymeric nanocarriers for cancer therapy, in Nano-Oncologicals: New Targeting and Delivery Approaches. ed. by B.M.H. Alonso, M. Garcia-Fuentes (Springer, Spain, 2014), pp.67–94
N.A. Atiyah, T.M. Albayati, M.A. Atiya, Functionalization of mesoporous MCM-41 for the delivery of curcumin as an anti-inflammatory therapy. Adv. Powder Technol. 33(2), 103417 (2022)
N. Singh, S. Son, J. An, I. Kim, M. Choi, N. Kong, W. Tao, J.S. Kim, Nanoscale porous organic polymers for drug delivery and advanced cancer theranostics. Chem. Soc. Rev. 50, 12883–12896 (2021)
S.B. Patil, S.Z. Inamdar, K.R. Reddy, A.V. Raghu, K.G. Akamanchi, A.C. Inamadar, K.K. Das, R.V. Kulkarni, Functionally tailored electro-sensitive poly (acrylamide)-g-pectin copolymer hydrogel for transdermal drug delivery application: synthesis, characterization, in-vitro and ex-vivo evaluation. Drug Deliv. Lett. 10(3), 85–196 (2020)
S. Mohapatra, S. Ranjan, N. Dasgupta, R. Mishra, S. Thomas, Nanocarriers carriers for drug delivery, 1st edn. (Elsevier, Amsterdam, 2018)
M. Meyyappan, Nanotechnology: opportunities and challenges. Electron. Today 37(9), 61–63 (2005)
P. Parhi, C. Mohanty, S.K. Sahoo, Nanotechnology-based combinational drug delivery: an emerging approach for cancer therapy. Drug Discovery Today 17(17–18), 1044–1052 (2012)
J. Nicolas, L. Moine, G. Barratt, Polymeric nanoparticles for drug delivery, in Polymeric Biomaterials, 1st edn., ed. by S. Dumitriu, V. Popa (CRC Press, Florida, 2013), pp.123–152
A. Zielińska, F. Carreiró, A.M. Oliveira, A. Neves, B. Pires, D.N. Venkatesh et al., Polymeric nanoparticles: production, characterization Toxicology and Ecotoxicology. Molecules 25(16), 3731 (2020)
S.Y. Lee, H.S. Park, K.Y. Lee, H.J. Kim, Y.J. Jeon, T.W. Jang et al., Paclitaxel-loaded polymeric micelle (230 mg/m(2)) and cisplatin (60 mg/m(2)) vs. paclitaxel (175 mg/m(2)) and cisplatin (60 mg/m(2)) in advanced non-small-cell lung cancer: a multicenter randomized phase iib trial. Clin. Lung Cancer 14(3), 275–282 (2013)
S.W. Lee, Y.M. Kim, C.H. Cho, Y.T. Kim, S.M. Kim, S.Y. Hur et al., An open-label, randomized, parallel, phase ii trial to evaluate the efficacy and safety of a cremophor-free polymeric micelle formulation of paclitaxel as first-line treatment for ovarian cancer: a korean gynecologic oncology group study (KGOG-3021). Cancer Res. Treat. 50(1), 195–203 (2018)
J.X. Zhang, M.Q. Yan, X.H. Li, L.Y. Qiu, X.D. Li, X.J. Li, Y. Jin et al., Local delivery of indomethacin to arthritis-bearing rats through polymeric micelles based on amphiphilic polyphosphazenes. Pharm. Res. 24, 1944–1953 (2007)
X. Wang, Xu. Bing Wei, J.W. Cheng, R. Tang, Phenylboronic acid-decorated gelatin nanoparticles for enhanced tumor targeting and penetration. Nanotechnology 27(38), 385101 (2016)
J.D. Twibanire, T.B. Grindley, Polyester dendrimers: smart carriers for drug delivery. Polymers 6(1), 179–213 (2014)
X. Ke, V.W. Ng, R.J. Ono, J.M. Chan, S. Krishnamurthy, Y. Wang et al., Role of non-covalent and covalent interactions in cargo loading capacity and stability of polymeric micelles. J. control. Releas. 193, 9–26 (2014)
T. Maeda, H. Otsuka, A. Takahara, Dynamic covalent polymers: reorganizable polymers with dynamic covalent bonds. Prog. Polym. Sci. 34(7), 581–604 (2009)
P. Gou, W. Liu, W. Mao, J. Tang, Y. Shen, M. Sui, Self-assembling doxorubicin prodrug forming nanoparticles for cancer chemotherapy: synthesis and anticancer study in vitro and in vivo. J. Mater. Chem. B 1(3), 284–292 (2013)
Y.E. Aguirre-Chagala, J.L. Santos, Y. Huang, M. Herrera-Alonso, Phenylboronic acid-installed polycarbonates for the ph-dependent release of diol-containing molecules. ACS Macro Lett. 3(12), 1249–1253 (2014)
Hu. Xianglong, Hu. Jinming, J. Tian, Z. Ge, G. Zhang, K. Luo et al., Polyprodrug amphiphiles: hierarchical assemblies for shape-regulated cellular internalization, trafficking, and drug delivery. J. Am. Chem. Soc. 135(46), 17617–17629 (2013)
A.C. Allison, R. Cacabelos, V.R. Lombardi, X.A. Alvarez, C. Vigo, Celastrol, a potent antioxidant and anti-inflammatory drug, as a possible treatment for Alzheimer’s disease. Prog. Neuropsychopharmacol. Biol. Psychiatry 25(7), 1341–1357 (2001)
A. Salminen, M. Lehtonen, T. Paimela, K. Kaarniranta, Celastrol: molecular targets of thunder god vine. Biochem. Biophys. Res. Commun. 394(3), 439–442 (2010)
H. Zhu, W.-J. Ding, Wu. Rui, Q.-J. Weng, J.-S. Lou, R.-J. Jin, Synergistic anti-cancer activity by the combination of TRAIL/APO-2L and celastrol. Cancer Invest. 28(1), 23–32 (2010)
S. Huang, Y. Tang, X. Cai, X. Peng, X. Liu, L. Zhang, Celastrol inhibits vasculogenesis by suppressing the VEGF-induced functional activity of bone barrow-derived endothelial progenitor cells. Biochem. Biophys. Res. Commun. 423(3), 467–472 (2012)
S.Y. Jang, S.W. Jang, J. Ko, Celastrol inhibits the growth of estrogen positive human breast cancer cells through modulation of estrogen receptorα. Cancer Lett. 300(1), 57–65 (2011)
P.-P. Li, W. He, P.-F. Yuan, S.-S. Song, Lu. Jing-Tao, W. Wei, Celastrol induces mitochondria-mediated apoptosis in hepatocellular carcinoma bel-7402 cells. Am. J. Chin. Med. 43(1), 137–148 (2015)
H. Ni, W. Zhao, X. Kong, H. Li, J. Ouyang, Celastrol inhibits lipopolysaccharide-induced angiogenesis by suppressing tlr4-triggered nuclear factor-kappa B activation. Acta Haematol. 131(2), 102–111 (2014)
V.R. Yadav, B. Sung, S. Prasad, R. Kannappan, S.G. Cho, M. Liu et al., Celastrol suppresses invasion of colon and pancreatic cancer cells through the downregulation of expression of CXCR4 chemokine receptor. J. Mol. Med. 88(12), 1243–1253 (2010)
Y. Kim, H. Kang, S.-W. Jang, J. Ko, Celastrol inhibits breast cancer cell invasion via suppression of NF-κB-mediated matrix metalloproteinase-9 expression. Cell. Physiol. Biochem. 28(2), 175–184 (2011)
X.H. Cai, J. Jin, M.H. He, 2016 Advances in structural modifications of celastrol. ARKIVOC 1, 172–182 (2016)
A. Trott, J.D. West, L. Klaić, S.D. Westerheide, R.B. Silverman, R.I. Morimoto et al., Activation of heat shock and antioxidant responses by the natural product celastrol: transcriptional signatures of a thiol-targeted molecule. Mol. Biol. Cell 19(3), 1104–1112 (2008)
J.H. Lee, T.H. Koo, H. Yoon, H.S. Jung, H.Z. Jin, K. Lee et al., Inhibition of NF-κB activation through targeting IκB kinase by celastrol, a quinone methide triterpenoid. Biochem. Pharmacol. 72(10), 1311–1321 (2006)
S. Sreeramulu, S.L. Gande, M. Göbel, H. Schwalbe, Molecular mechanism of inhibition of the human protein complex Hsp90-Cdc37, a kinome chaperone-cochaperone, by triterpene celastrol. Angewandte chemie-international edition. 48(32), 5853–5855 (2009)
P. Ashrit, B. Sadanandan, L. Kyathsandra Natraj, K. Shetty, V. Vaniyamparambath, A.V. Raghu, Microplate-based response surface methodology model for growth optimization and biofilm formation on polystyrene polymeric material in a Candida albicans and Escherichia coli co-culture. Polym. Adv. Technol. 33(9), 2872–2885 (2022)
G. Divyashri, T.P. Murthy, K.V. Ragavan, G.M. Sumukh, L.S. Sudha, S. Nishka, G. Himanshi, N. Misriya, B. Sharada, R.A. Venkataramanaiah, Valorization of coffee bean processing waste for the sustainable extraction of biologically active pectin. Heliyon. 9(9), e20212 (2023)
A.T. Khadim, T.M. Albayati, N.M. Saady, Removal of sulfur compounds from real diesel fuel employing the encapsulated mesoporous material adsorbent Co/MCM-41 in a fixed-bed column. Microporous Mesoporous Mat. 341, 112020 (2022)
N.S. Ali, Z.T. Alismaeel, H.S. Majdi, H.G. Salih, M.A. Abdulrahman, N.M. Saady, T.M. Albayati, Modification of SBA-15 mesoporous silica as an active heterogeneous catalyst for the hydroisomerization and hydrocracking of n-heptane. Heliyon 8(6), e09737 (2022)
N.S. Ali, N.M. Jabbar, S.M. Alardhi, H.S. Majdi, T.M. Albayati, Adsorption of methyl violet dye onto a prepared bio-adsorbent from date seeds: isotherm, kinetics, and thermodynamic studies. Heliyon. 8(8), e10276 (2022)
A.V. Raghu, H.M. Jeong, J.H. Kim, Y.R. Lee, Y.B. Cho, K. Sirsalmath, Synthesis and characterization of novel polyurethanes based on 4-{(4-Hydroxyphenyl)iminomethyl}phenol. Macromol. Res. 16(3), 194–199 (2008)
N.A. Atiyah, T.M. Albayati, M.A. Atiya, Interaction behavior of curcumin encapsulated onto functionalized SBA-15 as an efficient carrier and release in drug delivery. J. Mol. Struct. 1260(15), 132879 (2022)
N.S. Ali, H.N. Harharah, I.K. Salih, N.M. Cata Saady, S. Zendehboudi, T.M. Albayati, Applying MCM-48 mesoporous material, equilibrium, isotherm, and mechanism for the effective adsorption of 4-nitroaniline from wastewater. Sci. Rep. 13, 9837 (2023)
B. Salehi, Y. Berkay Yılmaz, G. Antika, T. Boyunegmez Tumer, M. Fawzi Mahomoodally, D. Lobine et al., Insights on the use of α-lipoic acid for therapeutic purposes. Biomolecules 9(8), 356 (2019)
M. Foox, M. Zilberman, Drug delivery from gelatin-based systems. Expert Opin. Drug Deliv. 12(9), 1547–1563 (2015)
Ş Yıldırım, H. Akyıldız, Z. Çetinkaya, Synthesis of glucose/fructose sensitive poly(ethylene glycol) methyl ether methacrylate particles with novel boronate ester bridge crosslinker and their dye release applications. Acta Chim. Slov. 69(1), 39–48 (2022)
Wu. Zhongyu, M. Li, H. Fang, B. Wang, A new boronic acid based fluorescent reporter for catechol. Bioorg. Med. Chem. Lett. 22(23), 7179–7182 (2012)
C. Wang, P. Qi, Lu. Yan, L. Liu, Y. Zhang, Q. Sheng et al., Bicomponent polymeric micelles for pH-controlled delivery of doxorubicin. Drug Deliv. 27(1), 344–357 (2020)
P. Zhang, Xu. Qinan, X. Li, Y. Wang, pH-responsive polydopamine nanoparticles for photothermally promoted gene delivery. Mat. Sci. Eng. C Mater. Biol. Appl. 108, 110396 (2020)
R. Mo, Gu. Zhen, Tumor microenvironment and intracellular signal-activated nanomaterials for anticancer drug delivery. Mater. Today 19, 274–283 (2016)
A.A. Cluntun, M.J. Lukey, R.A. Cerione, J.W. Locasale, Glutamine metabolism in cancer: Understanding the heterogeneity. Trends Cancer. 3(3), 169–180 (2017)
B. Sun, C. Luo, Yu. Han, X. Zhang, Q. Chen, W. Yang et al., Disulfide bond-driven oxidation- and reduction-responsive prodrug nanoassemblies for cancer therapy. Nano Lett. 18(6), 3643–3650 (2018)
M.H. Lee, J.L. Sessler, J.S. Kim, Disulfide-based multifunctional conjugates for targeted theranostic drug delivery. Acc. chem. Res.. 48(11), 2935–2946 (2015)
L. Zhang, Y. Ding, Q. Wen, C. Ni, Synthesis of core-crosslinked zwitterionic polymer nano aggregates and pH/Redox responsiveness in drug-controlled release. Mat. Sci. Eng. C Mater. Biol. Appl. 106, 110288 (2020)
J. Qin, Y. Huang, G. Yan, J. Wang, Hu. Liefeng, P. Zhang, R. Tang, Phenylboronic acid-functionalized ultra-pH-sensitivemicelles for enhanced tumor penetration and inhibitionin vitro. J. Mater. Sci. 54, 5695–5711 (2019)
Acknowledgements
This research was funded by the Collaborative Grant-in-Aid of the HBUT National “111” Center for Cellular Regulation and Molecular Pharmaceutics (XBTK-2021009) and Research Funds from Hubei University of Technology(GCRC20200013).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interests
The authors declare that there is no conflict of interests, we do not have any possible conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Su, J., Rao, M., Dai, H. et al. Dual stimuli-responsive polymeric prodrug consisting of reversible covalent bonded celastrol for tumor targeted delivery. Macromol. Res. 32, 173–186 (2024). https://doi.org/10.1007/s13233-023-00218-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13233-023-00218-6