Abstract
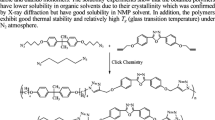
Although 2,2,4,4-tetramethyl-1,3-cyclobutadione (TMCBDI) has been known for more than 50 years, it has never been used for synthesis of polymer primarily due to its inefficiency in direct polycondensation reaction. We report the first synthesis of high molecular weight polyimines prepared from TMCBDI using Pd-catalyzed Suzuki coupling polymerization. To overcome sluggish condensation polymerization of TMCBDI, it was first converted to diimine monomer containing phenyl bromide on both sides (compound 1) via condensation reaction and polymerized with three different examples aryl diboronate compounds (2a, 2b, 2c). The resulting polyimines (3a, 3b, 3c) were found to exist in a mixture of E and Z isomers in a ratio of 2:1. The number and weight average molecular weights of polyimines were high considering the rigidity of the backbone structure: 20,000 and 44,300 g/mol and 23,100 and 40,500 g/mol for 3a and 3b, respectively. They also showed good thermal stability over 340 °C and a high glass transition temperature over 220 °C. There was no observable photoluminescence from the polyimines in the range of 800 to 300 nm. Apparently, the TMCBDI moiety disrupts electron delocalization in conjugated backbone of polymer chains.

Access this article
We’re sorry, something doesn't seem to be working properly.
Please try refreshing the page. If that doesn't work, please contact support so we can address the problem.
Similar content being viewed by others
References
R. Adams, J. E. Bullock, and W. C. Wilson, J. Am. Chem. Soc., 45, 512 (1923).
G. F. D'lelio, J. V. Crivello, R. K. Schoenig, and T. F. Huemmer, J. Macromol. Sci., Part A: Chem., A1, 1161 (1967).
P. W. Morgan, S. L. Kwolek, and T. C. Pletcher, Macromolecules, 20, 729 (1987).
K. S. Lee, J. C. Won, and J. C. Jung, Makromol. Chem., 190, 1547 (1989).
K. S. Lee and M. Samoc, Polym. Commun., 32, 361 (1991).
C. J. Yang and S. A. Jenekhe, Chem. Mater., 3, 878 (1991).
C. J. Yang and S. A. Jenekhe, Macromolecules, 28, 1180 (1995).
S. Barik and W. G. Skene, Polym. Chem., 2, 1091 (2011).
C. I. Simionescu, M. Grigoras, I. Cianga, and N. Olaru, Eur. Polym. J., 34, 891 (1998).
M. Grigoras, I. Cianga, A. Farcas, G. Nastase, and M. Ivanoiu, Rev. Roum. Chim., 45, 703 (2000).
R. Gauderon, C. J. G. Plummer, J. G. Hilborn, and D. M. Knauss, Macromolecules 31, 501 (1998).
M. Grigoras and N. C. Antonoaia, Polym. Int., 54, 1641 (2005).
I. Mihai, M. Ivanoiu, L. Vacareanu, and M. Grigoras, Des. Monomers Polym., 15, 41 (2012).
F. R. Diaz, J. Moreno, L. H. Tagle, G. A. East, and D. Radic, Synth. Met., 100, 187 (1999).
R. H. Hasek, E. U. Elam, and J. C. Martin, J. Org. Chem., 26, 4340 (1961).
J. J. Worman and E. A. Schmidt, J. Org. Chem., 35, 2463 (1970).
J. M. Behan, R. A. W. Johnstone, J. J. Worman, and T. P. Fehlner, J. Mol. Struct., 40, 151 (1977).
A. Naik, M. Ferro, and J. Worman, Org. Lett., 4, 1059 (2002).
N. Miyaura and A. Suzuki, Chem. Rev., 95, 2457 (1995).
M.-J. Park, J. Lee, I. H. Jung, J.-H. Park, H. Kong, J.-Y. Oh, D.-H. Hwang, and H.-K. Shim, J. Polym. Sci., Part A: Polym. Chem., 48, 82 (2010).
A. Iwan and D. Sek, Prog. Polym. Sci., 33, 289 (2008).
R. Tanoue, R. Higuchi, N. Enoki, Y. Miyasato, S. Uemura, N. Kimizuka, A. Z. Stieg, J. K. Gimzewski, and M. Kunitake, ACS Nano, 5, 3923 (2011).
B. J. Landi, C. M. Evans, J. J. Worman, S. L. Castro, S. G. Bailey, and R. P. Raffaelle, Mater. Lett., 60, 3502 (2006).
M. K. Choi, H. L. Kim, and D. H. Suh, J. Appl. Polym. Sci., 101, 1228 (2006).
H. J. Kim, J. H. Lee, M. Lee, and T. S. Lee, React. Funct. Polym., 68, 1696 (2008).
S. Clavaguera et al., J. Polym. Sci., Part A: Polym. Chem., 47, 4141 (2009).
D. Urselmann, D. Antovic, and T. J. J. Muller, Beilstein J. Org. Chem., 7, 1499 (2011).
U. Rant, U. Scherf, M. Rehahn, P. Galda, J. L. Brédas, and E. Zojer, Synth. Met., 127, 241 (2002).
S. H. Jung, T. W. Lee, Y. C. Kim, D. H. Suh, and H. N. Cho, Opt. Mater., 21, 169 (2002).
Author information
Authors and Affiliations
Corresponding author
Additional information
Acknowledgments: The authors gratefully acknowledge NSF grant (DMR-0747667) and Rensselaer Polytechnic Institute (start-up) for generous support and Prof. James C. Selser (Department of Physics and Astronomy, University of Nevada Las Vegas) for use of DSC/TGA instruments.
Rights and permissions
About this article
Cite this article
Lee, YB., Lee, WH., Worman, J.J. et al. Synthesis and property of polyimines containing 2,2,4,4-tetramethyl-1,3-cyclobutadiimine moiety. Macromol. Res. 25, 578–583 (2017). https://doi.org/10.1007/s13233-017-5119-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13233-017-5119-4