Abstract
Background
Tibolone is an alternative to conventional estrogen and progesterone in relieving post-menopausal symptoms in Indian women.
Material and Methods
A prospective short-term observational study was done at a tertiary care teaching hospital in New Delhi from November 2019 to September 2021. Fifty-three women, less than 60 years of age, presenting with moderate to severe intensity of menopausal symptoms as assessed by measuring menopausal rating score (MRS > 8) were enrolled and given Tibolone 2.5 mg daily for 3 months. Improvements in symptoms were seen at 1 month and 3 months. Side effects were also noted.
Results
Marked improvement was seen as reduction in scores of psychological, somatic and genitourinary symptoms was noted. The psychological symptoms reduced from 8.92 ± 1.959 to 2.905 ± 1.042, the somatic symptoms decreased from 8.33 ± 2.299 to 3.4 ± 1.167, and genitourinary symptoms decreased from 3.64 ± 1.42 to 2.150 ± 0.948 after 3 months of treatment with Tibolone. Only 3 patients (5.6%) experienced vaginal spotting with no major side effects.
Conclusions
Tibolone is a highly effective and well accepted drug to reduce moderate to severe menopausal symptoms, especially psychological symptoms including depression.
Similar content being viewed by others
Introduction
Menopause is a naturally occurring event in all women after the reproductive age. The menopausal symptoms adversely affect the quality of life in menopausal women. Hormonal replacement therapy helps to alleviate the bothersome symptoms. Tibolone, which is a STEAR (Selective Tissue Estrogenic Activity Regulator), is used for the treatment of women with menopausal symptoms. It possesses estrogenic, progestogenic, and androgenic properties [1]. This study was done to see the effectiveness of Tibolone in alleviating post-menopausal symptoms in Indian women.
Materials and Methods
A short-term prospective observational study was conducted in the department of obstetrics and gynecology, from November 2019 to September 2021. Inclusion criteria were women with age less than 60 years having moderate to severe intensity of menopausal symptoms as assessed by Menopausal Rating Score (MRS > 8). The exclusion criteria were postmenopausal women with endometrial thickness greater than 4 mm, women with breast cancer or family history of breast cancer, severe medical disorders (cardiac, liver, renal diseases), undiagnosed vaginal bleeding, history of psychiatric illness diagnosed before menopause, and women with an active or past history of thromboembolic disorders.
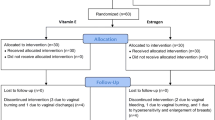
A total of 150 patients attending menopausal OPD were screened for enrollment, which included a detailed history, examination, and ultrasound evaluation for endometrial thickness. Out of these, 76 patients who met the inclusion criteria were enrolled in the study. Enrolled patients were given Tibolone 2.5 mg once daily for a period of 3 months and were assessed for improvement in menopausal symptoms objectively by MRS questionnaire at 1 month and 3 months after taking Tibolone. Side effects at 15 days, 1 month, and 3 months were noted.
Observations and Results
Patients presenting to menopause OPD and gynecology OPD were assessed for the severity of menopausal symptoms. Patients meeting exclusion criteria and those with mild severity scores (MRS score < 8) were excluded from the study. Seventy-six patients were enrolled in this study, and among them, 23 patients (30.2%) were lost to follow-up (due to the Covid-19 pandemic, which occurred during the duration of our study period) and were not included in the study. Fifty-three patients completed the 3 month treatment with Tibolone.
Demographic Variables of the Study Population
The age of patients in the study population ranged from 39 to 59 years, with a mean age of 51.2 years. Thirty-seven patients (69.8%) were in the age group of 50–60 years, 15 patients (28.3%) were in the age group of 40–50 years, and only one patient (1.8%) was in the age group of 30–40 years. The age at menopause in the study population ranged from 36 to 54 years. The mean age at menopause was 46.79 years. There was only one patient among the study population, with premature ovarian failure with menopause at 36 years. The duration of menopause ranged from 1 to 14 years in the study population, with a mean duration of menopause of 4.6 years with a SD of 3.05 years. Thirty-eight patients (71.69%) had their menopause for duration of 1–6 years, 13 patients (24.52%) had their menopause for a duration of 7–12 years, and 2 patients (3.7%) had their menopause for a duration of 13–18 years. In the study population, 48 patients (90.5%) had natural menopause, while 5 patients (9.4%) had surgical menopause.
Among the study population, 33 patients (62%) belonged to the lower middle class. Only 1 patient (1.8%) belonged to the lower class, 4 patients (7.5%) belonged to the upper lower class, 12 patients (23%) belonged to upper middle class and 3 patients (5.6%) belonged to upper class.
Effect of Tibolone on Severity of Menopausal Symptoms
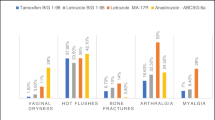
At enrollment, 11 patients (20.75%) had a total MRS score in the moderate severity range (9–15) and 42 patients (79.42%) had a total MRS score in the severe range (> 15). There was a marked reduction in the severity of symptoms after 1 month and 3 months of taking Tibolone (Fig. 1). No patient reported severe intensity of symptoms at the end of 3 months of taking Tibolone. The mean of total MRS score at enrollment was 21 with SD of 4.5. This reduced it to 13.98 with a SD of 2.196 after 1 month of taking Tibolone. After 3 months of taking Tibolone, the mean of the total score further decreased to 8.47 with a SD of 2.09 (Fig. 2). This difference was statistically significant with a p value of < 0.00001 both at 1 month and 3 months of taking Tibolone.
Effect of Tibolone on Psychological Symptoms
The psychological symptoms at enrollment ranged from 5 to 12 with a mean score of 8.92 and a SD of 1.95. This was decreased to 5.622 with a SD of 1.259 after 1 month of taking Tibolone and further to 2.905 with a SD of 1.042 after 3 months of taking Tibolone (Fig. 3). This improvement was found to be highly statistically significant with a p value of < 0.00001 both at 1 month and 3 months of taking Tibolone. 7 patients (13.2%), 18 patients (33.9%), and 23 patients (43.3%) who reported very severe, severe and moderate intensity of depression, respectively, at enrollment, had significant improvement in their symptoms with none of the patients reporting severe or very severe intensity, while only 1 patient (1.8%) reported moderate intensity of depression at 3 months of taking Tibolone.
Effect of Tibolone on Somatic Symptoms
At enrollment, the somatic subscore ranged from 4 to 12 with a mean score of 8.33 with a SD of 2.29, which reduced to 5.4 with a SD of 1.39 after 1 month of taking Tibolone and further to 3.4 with a SD of 1.16 after 3 months of taking Tibolone (Fig. 4). This difference was found to be statistically significant with a p value of < 0.00001, both at 1 month and 3 months of treatment with Tibolone.
At enrollment, 63% of patients reported hot flushes of varying intensity. Fifteen patients (28.3%), 13 patients (24.5%), 4 patients (7.54%) and 1 patient (1.8%) reported very severe, severe, moderate and mild intensity of hot flushes, respectively. These patients had improvement in their symptom intensity after 1 month of taking Tibolone, with 4 patients (7.54%) reporting complete resolution of hot flushes and none of the patients reported very severe intensity of hot flushes. Only 3 patients (5.6%) reported severe intensity. After 3 months of taking Tibolone, a total of 11 patients (20.75%) reported having complete resolution of hot flushes and none of the patients had severe or very severe intensity of hot flushes (Fig. 5).
Effect of Tibolone on Genitourinary Symptoms
The Genitourinary subscore at enrollment ranged from 1–9 with a mean score of 3.64 and a SD of 1.42. This decreased to 2.867 with a SD of 0.832 after 1 month of taking Tibolone and further decreased to 2.150 with a SD of 0.948 after 3 months of taking Tibolone (Fig. 6).
Effect of Tibolone on Endometrial Thickness
The endometrial thickness at enrollment was 2.6 ± 0.53 mm. It was reassessed after 3 months of treatment with Tibolone, and it was observed that the mean value decreased to 2.19 mm with a SD of 0.44 mm. The difference in the mean values was found to be significant (p < 0.00001).
Side Effects with Tibolone
The patients were assessed for side effects at 15 days, 1 month, and 3 months after starting treatment with Tibolone. Only 3 patients (5.6%) were reported to have vaginal spotting, 2 of them in the initial 15 days of taking Tibolone. 3rd patient reported the same after 1 month of taking Tibolone. All were isolated incidents that resolved spontaneously and were not reported to be bothersome by the patients. None of the patients in the study reported having breast tenderness, calf pain or other symptoms of DVT at 15 days, 1 month, or at 3 months of taking Tibolone (Table 1).
Effect on LFT and Lipid Profile
Lipid profile and liver function tests were assessed at enrollment, and after 3 months of taking Tibolone, no significant statistical difference was observed. After 3 months of treatment, the mean total bilirubin decreased to 0.635 mg/dl, the mean SGOT increased, and the SGPT value decreased. These differences were not significant statistically (p = 0.176, 0.412, 0.17, respectively). The effect on the lipid profile showed no statistical difference. An increase in total VLDL cholesterol was seen from a mean of 33 mg/dl to33.4 mg/dl, but was not statistically different (p = 0.502). There was a non-significant reduction in total cholesterol, HDL, LDL, and TG with p values of 0.09, 0.25, 0.21 and 0.31, respectively.
Discussion
There has been a resurgence of interest in treatment for postmenopausal symptoms, which can be distressing and reduce the quality of life. A relook at WHI study and as per recommendations of international menopausal society in 2016 [2, 3], selection of patients less than 60 years of age reduces the adverse effects of hormonal replacement therapy. Tibolone can be a safer alternative to the conventional estrogen and progesterone treatments as its pharmacodynamic profile is significantly different. It is synthetic steroid which is a Selective Tissue Estrogen Activity Regulator (STEAR). Upon oral ingestion, it is converted into 3 α hydroxyl Tibolone, 3 β hydroxy Tibolone and δ4 isomer. The hydroxy metabolites are estrogenic and have a positive effect on vagina and bone homeostasis. The delta isomer is responsible for both the progestogenic as well as androgenic properties of Tibolone. The progestogenic action is responsible for the protective effect of Tibolone in endometrium, while the androgenic action is responsible for the reduction in vasomotor symptoms of hot flushes and enhancing the sexual well being [4, 5]. The major advantage of Tibolone and its hydroxy metabolites is their lack of estrogenic activity in breast tissue, mostly by inhibiting the enzyme estrogen sulfatase and partly by increasing the enzyme activity of sulfatotransferase thereby producing the inactive metabolite-estrogen sulfate and effectively decreasing the active estrogen-estradiol thus decreasing incidence of breast cancer.
In our study population, 33 patients (62%) presented with symptoms of hot flushes. Indian studies find hot flushes less common and less bothersome. An incidence of 36.7% was found in rural India [6] and 34% in urban India [7]. Western countries report a higher incidence (80% to 88%) of postmenopausal hot flushes [8, 9]. At the end of three months of taking Tibolone, patients reported significant improvement in the hot flushes as there were no patients who reported very severe or severe intensity of hot flushes. Only 8 patients (15%) reported moderate hot flushes; 14 patients reported only mild hot flushes; and 31 patients (58.4%) reported complete resolution of hot flushes. This effect of Tibolone was similar to the Cochrane review and other studies, which spanned from three months to three years [1, 4, 5, 8, 9].
The mean total MRS score decreased to 8.47 ± 2.09 after 3 months of treatment with Tibolone. Somatic, psychological, and genitourinary symptoms significantly improved from the baseline, with maximum reduction in symptoms seen in the psychological subscale, followed by somatic and then genitourinary symptoms.
As seen in our study, Tibolone is a well-tolerated drug. In our study, only 5.6% (n = 3) reported a single self-limiting episode of vaginal spotting. These results were lower when compared to the THEBES trial, in which vaginal bleeding was 14.5%. The Cochrane review also reported an incidence of 31–44% [1]. This could be explained as our inclusion criteria in patients was endometrial thickness less than 4 mm, unlike other studies. No significant change in endometrial thickness with Tibolone was noted in our study, which is similar to that reported in the OPAL study [10]. During treatment with Tibolone, no effect on liver function tests or lipid profile was noted in our study. This effect on lipid profile differs from the results of a meta-analysis in which they reported a decrease in total cholesterol, HDL-C and triglyceride levels [11–13]. However, they had included studies in which Tibolone was given for a longer duration in contrast to our study where Tibolone was given only for a period of 3 months. Tibolone given for longer period has been shown to cause elevated liver enzymes [14, 15].
There are few studies using Tibolone for relief of menopausal symptoms in India. Authors found a short trial of three months of Tibolone helps menopausal patients tide over, offering a marked reduction in symptoms and an improved quality of life with no side effects, but patients need reassessment for prolonged use. The ease of drug usage, daily dosing compared to sequential estrogen and progesterone in patients with an intact uterus is an advantage for patient compliance. The short coming of our study was the short duration of 3 months. As the symptoms are seen in the transition phase too, Tibolone can be given for short term, though in our study we restricted its use in menopausal patients. The effect of Tibolone on bone health was not assessed because of the short-term nature of our study. The beneficial effect is seen in prevention, although Tibolone is not recommended for treatment of osteopenia under current guidelines. Tibolone is also a drug of choice in patients who had endometriosis [16] although no patient in our study had endometriosis. A short-term treatment is highly effective in reducing distressing symptoms of menopause, improving quality of life with no side effects. Patients should be re-assessed after three months and re-evaluated for long-term treatment in case of recurrence symptoms.
Conclusion
Tibolone is a highly effective and well accepted drug to reduce moderate to severe menopausal symptoms, especially psychological symptoms including depression. The maximum reduction was seen in the psychological symptoms, followed by somatic and genitourinary symptoms. A short three-month treatment relieves most of the symptoms without any side effects; long-term treatment needs further monitoring.
References
Giulio F, Enrica P, Susanna M, et al. Short-term and long term effects of tibolone in postmenopausal women, Cochrane review. Cochrane Database Syst Rev. 2016;10:CD008536.
Baber RJ, Panay N, Fenton A, IMS Writing Group. IMS Recommendations on women’s midlife health and menopause hormone therapy. Climacteric. 2016;19(2):109–50. https://doi.org/10.31019/13697137.2015.1129166.
Amy V, Rebecca D, Jennifer K. Menopause: a global perspective and clinical guide for practice. Clin Obstet Gynecol. 2021;64(3):528–54.
Bansal R, Aggarwal N. Menopausal hot flashes: a concise review. J Mid Life Health. 2019;10(1):6–13. https://doi.org/10.4103/jmh.JMH_7_19.
Jane FM, Davis SR. A practitioner’s toolkit for managing the menopause. Climacteric. 2014;17(5):564–79. https://doi.org/10.3109/13697137.2014.929651.
Meenakshi K, Komal S, Priyanka C, Seema V, Pankaj K, Tarun S. Prevalence of menopausal symptoms and its effect on quality of life among rural middle aged women (40–60 years) of Haryana, India [internet].org. Int J Appl Basic Med Res. 2020;10(3):183–8. https://doi.org/10.4103/ijabmr.IJABMR_428_19.
Malik R, Pokeria C, Singh S. Correlation of menopausal symptoms with serum estradiol: a study in urban Indian postmenopausal women. J Obstet Gynecol India. 2021. https://doi.org/10.1007/s13224-021-01518-6.
Huang KE, Baber R, Pacific A, Tibolone Consensus Group. Updated clinical recommendations for the use of tibolone in Asian women. Climacteric. 2010;13(4):317–27. https://doi.org/10.3109/13697131003681458.
Landgren MB, Helmond FA, Engelen S. Tibolone relieves climacteric symptoms in highly symptomatic women with at least seven hot flushes and sweats per day. Maturitas. 2005;50(3):222–30. https://doi.org/10.1016/j.maturitas.2004.06.001.
Changu LV, Zhang W, Tan X, Shang X, Găman MA, Salem H, et al. The effect of Tibolone treatment on lipid profile in women: a systematic review and dose–response meta-analysis of randomized controlled trials. Pharmacol Res. 2021;169:105612. https://doi.org/10.1016/j.phrs.2021.105612.
Langer RD, Landgren BM, Rymer J, Helmond FA, OPAL Investigators. Effects of Tibolone and continuous combined conjugated equine estrogen/medroxyprogesterone acetate on the endometrium and vaginal bleeding: results of the OPAL study. Am J Obstet Gynecol. 2006;195(5):1320–7. https://doi.org/10.1016/j.ajog.2006.03.045.
Nieciecka A, Kędziora-Kornatowska K, Janiszewska M. Tibolone among drugs in the therapy of postmenopausal women. Med Res J. 2021;6:140–6.
Kotani K, Sahebkar A, Serban C, Andrica F, Toth PP, Jones SR, et al. Lipid and blood pressure meta-analysis collaboration (LBPMC) group. Tibolone decreases lipoprotein (a) levels in postmenopausal women. Atherosclerosis. 2015;242(1):87–96. https://doi.org/10.1016/j.atherosclerosis.2015.06.056.
Fait T. Menopause hormone therapy: latest developments and clinical practice. Drugs Context. 2019;2(8): 212551. https://doi.org/10.7573/dic.212551.
LiverTox. Clinical and research information on drug-induced liver injury [internet]. Bethesda: National Institute of Diabetes and Digestive and Kidney Diseases; 2012-. Tibolone. [Updated Sep 2 2020].
Gemmell LC, Webster KE, Kirtley S, Vincent K, Zondervan KT, Becker CM. The management of menopause in women with a history of endometriosis: a systematic review. Hum Reprod Update. 2017;23(4):481–500. https://doi.org/10.1093/humrupd/dmx011.
Funding
None.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
None.
Ethical Approval
Ethical approval was taken from Institutional Ethical committee-FP. No TP(MD/MS)()/IEC/ABVIMS/RMLH736/19.
Ethical Standards
This study was performed in line with the principles of the Declaration of Helsinki.
Informed Consent
Written informed consent was taken from all patients for participation and publication.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Dr. Renuka Malik is working as a consultant OB-GYN in ABVIMS and Dr. RML Hospital, New Delhi. She is an alumni of Lady Hardinge Hospital, New Delhi. Her areas of interest include high-risk obstetrics and menopause. She runs the menopause clinic at her institution. Dr. P. Meghana Reddy is Resident, OB-GYN, ABVIMS & Dr. RML Hospital, New Delhi.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Malik, R., Meghana Reddy, P. Effectiveness of Tibolone in Relieving Postmenopausal Symptoms for a Short-Term Period in Indian Women. J Obstet Gynecol India 73, 242–247 (2023). https://doi.org/10.1007/s13224-022-01727-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13224-022-01727-7