Abstract
Objectives
This prospective clinical trial was conducted to assess serum bile acids (BA) levels in women with intrahepatic cholestasis of pregnancy (ICP) compared to both pregnant and non-pregnant controls; and evaluate perinatal outcome in relation to bile acid levels. A scoring is proposed based on biochemical markers to optimize management in ICP cases.
Materials and Methods
Serum bile-acids(BA) were assessed in 71 intrahepatic-cholestasis of pregnancy(ICP) cases (group-I), versus 50 pregnant (group-II) and 35 non-pregnant (group-III) controls. Ursodeoxycholic acid (UDCA) was administered in ICP group. Baseline bilirubin (SB), aminotransferases (AT), alkaline-phosphatase were sent in groups I & II. Investigations were repeated in group-I after 4 weeks. Perinatal complications were noted.
Results
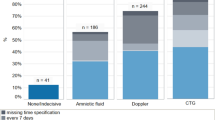
Mean BA in group-I was 75.92 ± 39.9 µmol/L which reduced to 41.3 ± 15.4 µmol/L(45.6%, p < 0.001) with UDCA. Mean BA was 29.2 ± 5.7 and 5.9 ± 1.8 µmol/L in group-II and group-III. UDCA significantly reduced itching-score. Rate of fetal distress linearly increased with the increasing baseline levels of serum BA, AT and SB: from 2.5 to 100% at BA < 40 and ≥ 200 µmol/L, (p = 0.008); from 16.1 to 100% at AT < 100 and ≥ 500 IU/mL(p = 0.016); and from 6.8 to 100% at SB < 0.8 and > 5 mg/dL (p = 0.001); respectively. Their baseline levels were divided into 5 groups in correlation to fetal distress. Serum BA < 40, 40–80, 80–120, 120–200, ≥ 200 µmol/L; AT < 100,100–200,200–500, ≥ 500 IU/mL; and SB < 0.8, 0.8–1.0, 1.1–2, 2.1–5 and > 5 mg/dL. Nutan ICP scoring was proposed with a score 0 to 4 given to each parameter and score-based management protocol was suggested for fetal surveillance and delivery.
Conclusions
SBA are higher in Asian Indian pregnant women. Levels > 30 µmol/L can be taken as a cut off for diagnosing ICP in Asian-Indian women. Adopting higher cut-offs for this geographic part will avoid over-diagnosing ICP and iatrogenic early termination of pregnancy. Suggested scoring will help clinicians in optimizing the time of delivery on an individualized basis.
Similar content being viewed by others
References
Geenes V, Williamson C. Intrahepatic cholestasis of pregnancy. World J Gastroenterol. 2009;15:2049–66.
Smith DD, Rood KM. Intrahepatic cholestasis of pregnancy. Clin Obstet Gynecol. 2020;63(1):134–51.
Piechota J, Jelski W. 2020 Intrahepatic cholestasis in pregnancy: review of the literature. J Clin Med. 6:9(5):E1361.
Glantz A, Marschall HU, Mattsson LA. Intrahepatic cholestasis of pregnancy: relationships between bile acid levels and fetal complication rates. Hepatology. 2004;40(2):467–74.
Castaño G, Lucangioli S, Sookoian S, Mesquida M, Lemberg A, Di Scala M, et al. Bile acid profiles by capillary electrophoresis in intrahepatic cholestasis of pregnancy. Clin Sci (Lond). 2006;110(4):459–65.
Palma J, Reyes H, Ribalta J, Hernández I, Sandoval L, Almuna R, et al. Ursodeoxycholic acid in the treatment of cholestasis of pregnancy: a randomized, double-blind study controlled with placebo. J Hepatol. 1997;27(6):1022–8.
Kenyon AP, Tribe RM, Nelson-Piercy C, Girling JC, Williamson C, Seed PT, et al. Pruritus in pregnancy: a study of anatomical distribution and prevalence in relation to the development of obstetric cholestasis. Obstet Med. 2010;3:25–9.
Reyes H, Gonzalez MC, Ribalta J, Aburto H, Matus C, Schramm G, et al. Prevalence of intrahepatic cholestasis of pregnancy in Chile. Ann Intern Med. 1978;88(4):487–93.
Sharma N, Panda S, Singh AS. Obstetric outcome during an era of active management for obstetrics cholestasis. J Obstet Gynecol India. 2016;66:38–41.
Hafeez M, Ansari A, Parveen S, Salamat A, Aijaz A. Frequency of intrahepatic cholestasis of pregnancy in Punjab Pakistan: a single centre study. J Pak Med Assoc. 2016;66(2):203–6.
Geenes V, Chappell LC, Seed PT, Steer PJ, Knight M, Williamson C. 2014 Association of severe intrahepatic cholestasis of pregnancy with adverse pregnancy outcomes: a prospective population-based case-control study. Hepatology;59(4-:1482–91.
Zhao CQ, Shao Y, Wu WX. Study on clinical grading of intrahepatic cholestasis of pregnancy. J Pract Obstet Gynecol. 1999;15(4):199–200.
Chen J, Deng W, Wang J, Shao Y, Ou M, Ding M. Primary bile acids as potential biomarkers for the clinical grading of intrahepatic cholestasis of pregnancy. Int J Gynaecol Obstet. 2013;122(1):5–8.
Rook M, Vargas J, Caughey A, Bacchetti P, Rosenthal P, Bull L. Fetal outcomes in pregnancies complicated by intrahepatic cholestasis of pregnancy in a Northern California cohort. PLoS ONE. 2012;7(3):e28343.
Brouwers L, Koster MP, Page-Christiaens GC, Kemperman H, Boon J, Evers IM, et al. Intrahepatic cholestasis of pregnancy: maternal and fetal outcomes associated with elevated bile acid levels. Am J Obstet Gynecol. 2015;212(1):100.e1-7.
Kawakita T, Parikh LI, Ramsey PS, Huang CC, Zeymo A, Fernandez M, et al. Predictors of adverse neonatal outcomes in intrahepatic cholestasis of pregnancy. Am J Obstet Gynecol. 2015;213(4):570.e1-8.
Bacq Y, Sentilhes L, Reyes HB, Glantz A, Kondrackiene J, Binder T, et al. Efficacy of ursodeoxycholic acid in treating intrahepatic cholestasis of pregnancy: a meta-analysis. Gastroenterology. 2012;143(6):1492–501.
Kong X, Kong Y, Zhang F, Wang T, Yan J. Medicine (Baltimore). Evaluating the Effectiveness and Safety of Ursodeoxycholic Acid in Treatment of Intrahepatic Cholestasis of Pregnancy: A Meta-Analysis. (A Prisma-Compliant Study). 2016;95(40):e4949.
Cui D, Zhong Y, Zhang L, Du H. Bile acid levels and risk of adverse perinatal outcomes in intrahepatic cholestasis of pregnancy: A meta-analysis. J Obstet Gynaecol Res. 2017;43(9):1411-20.
Di Mascio D, Quist-Nelson J, Riegel M, George B, Saccone G, Brun R, et al. Perinatal death by bile acid levels in intrahepatic cholestasis of pregnancy: a systematic review. J Matern Fetal Neonatal Med. 2019;1–9.
Ovadia C, Seed PT, Sklavounos A, Geenes V, Di Ilio C, Chambers J, et al. Association of adverse perinatal outcomes intrahepatic cholestasis of pregnancy with biochemical markers: results of aggregate and individual patient data meta-analyses. Lancet. 2019;393(10174):899–909.
Grand’Maison S, Durand M, Mahone M. The effects of ursodeoxycholic acid treatment for intrahepatic cholestasis of pregnancy on maternal and fetal outcomes: a meta-analysis including non-randomized studies. J Obstet Gynaecol Can. 2014;36(7):632–41.
Chappell LC, Bell JL, Smith A, Linsell L, Juszczak E, Dixon PH, PITCHES study group, et al. Ursodeoxycholic acid versus placebo in women with intrahepatic cholestasis of pregnancy (PITCHES): a randomised controlled trial. Lancet. 2019;394(10201):849–60.
Williamson C, Miragoli M, Sheikh Abdul Kadir S, et al. Bile acid signaling in fetal tissues: implications for intrahepatic cholestasis of pregnancy. Dig Dis. 2011;29(1):58–61.
Puljic A, Kim E, Page J, Esakoff T, Shaffer B, LaCoursiere DY, et al. Am J Obstet Gynecol. 2015;212(5):667.e1-5.
Acknowledgements
The project was funded by intramural grant of the institute, All India Institute of Medical Sciences, New Delhi
Funding
Intramural grant from All India Institute of Medical Sciences, New Delhi, India.
Author information
Authors and Affiliations
Contributions
All authors contributed in the patient management and follow-up. NA conceptualized the study. RM and VS were responsible for the acquisition of clinical data, and obtaining informed consent. NA, VK and RM were responsible for manuscript writing. NA, AK and AS had overall supervision. NA and VK were responsible for manuscript’s critical editing.
Corresponding author
Ethics declarations
Conflict of interest
None of the authors have any potential conflict of interest.
Ethical statement
Study was conducted after obtaining approval from the Institute’s Ethics Committee.
Human Participants and/or Animals
All parts of Declaration of Helsinki have been applied.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Nutan Agarwal is an Ex-Professor in Department of Obstetrics and Gynaecology, All India Institute of Medical Sciences, New Delhi, India. Reeta Mahey is an Additional Professor, Department of Obstetrics and Gynaecology, All India Institute of Medical Sciences, New Delhi. Vidushi Kulshrestha is an Associate Professor at Department of Obstetrics and Gynaecology, All India Institute of Medical Sciences, New Delhi. Alka Kriplani is an Ex-Professor in Department of Obstetrics and Gynaecology, All India Institute of Medical Sciences, New Delhi, India. Anoop Saraya is a Professor in Department of Gastroenterology, All India Institute of Medical Sciences, New Delhi, India. Vikas Sachdev is a Senior Technician in Department of Gastroenterology, All India Institute of Medical Sciences, New Delhi, India.
Rights and permissions
About this article
Cite this article
Agarwal, N., Mahey, R., Kulshrestha, V. et al. Serum Bile Acids in Intrahepatic Cholestasis of Pregnancy (ICP), Versus Pregnant and Nonpregnant Controls in Asian Indian Women and a Proposed Scoring to Optimize Management in ICP. J Obstet Gynecol India 72, 218–224 (2022). https://doi.org/10.1007/s13224-021-01501-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13224-021-01501-1