Abstract
Since many microfluidic devices have limited drug dose order of gradients and incorporate 2D cell culture, we here present a multi-layered platform with linear and logarithmic gradients with 3D-cell culturing chambers. By employing Hagen–Poiseuille flow resistance equation and the parallel electric schematics, we determined the appropriate channel dimensions to achieve the desired target concentrations (100%, 50%, 20%, 10%, 5%, 2%, 1%, 0%). To validate the gradient formation against theoretical values, we introduced a solution containing fluorescein into the microfluidic chip. Moreover, cell culturing chambers were spaced out laterally for every 9 mm, aligning with the dimensions with the standard plate reader, providing enhanced usability. Vertical layout of the chip minimized the lateral dimension required for housing various components while maintaining a favorable height for imaging. By preventing the need to use external tubing to connect concentration gradient generator and cell culturing chamber modules, our platform holds promise in facilitating the integration of microfluidics into drug evaluation processes. To demonstrate use of this flexible platform, we tested two chemotherapy drugs against human bladder cancer cells (T24) embedded in 3D fibrin gel and evaluated their cell viability and proliferation rate. IC50 values were extracted for cells exposed to varying doses of cisplatin, gemcitabine, and gemcitabine with a fixed cisplatin dose, confirming the enhanced apoptosis of the bladder cancer cells and the advantages of combination chemotherapy. This simple multi-layered device may accelerate screening of anti-cancer drugs for a specific cell type by extracting optimal dosage for two drugs.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Despite decades of cancer research and a majority of medical research funding being allocated to it, cancer remains an unresolved medical condition in our society [1]. Cancer is the second leading cause of death and is projected to remain a prominent health issue due to the aging population [2]. Since WHO projects up to 13.1 million deaths will be linked to cancer by 2030, cancer is a critical medical condition that must be addressed [3, 4]. Even though the first-line of treatment for many cancer patients is still surgery, numerous advancements in neoadjuvant and adjuvant administration have made chemotherapy the most prominent treatment option. Over the years, in addition to conventional chemotherapy, there have been exciting advancements in cancer therapies such as immunotherapy and targeted therapy [1, 5]. To optimize clinical outcomes of various cancer patients, engineers can develop a platform that can efficiently screen for best drug and dosage for specific cancer types to fully utilize innovative cancer therapies. To make the best use of these anti-cancer therapeutics, there needs to be a systematic way of screening them in vitro in a controlled manner.
Among various experimental parameters that influence cell viability, drug dose is the most influential one, which signals an urgent need for a drug screening platform with a comprehensive range of drug dosage, with both linear and logarithmic gradients [6]. Moreover, considering that combination chemotherapy is commonly used for treating cancer patients in the clinical setting due to cytotoxicity and drug resistance, there have also been an increasing demand for screening multiple types of drugs in a single experiment [7]. In other words, it is important to have a multi-drug screening platform that will enable testing various dose combinations of the drug candidates and their optimal dosage. Since cancer is gradually being accepted as a chronic disease not a fatal one, the shift towards personalized medicine and prioritizing the quality of care to minimize drug side effects is accelerating the demand for a highly efficient multi-drug screening process.
In the preclinical stage, conventional drug screening is conducted on 96-well plates with liquid-handling robots; this process is efficient but compromises physiological relevance with limited 2D cell monolayer cultures [8]. The dilemma lies where screening on well-plates with in vitro model is automated but inaccurate, whereas in vivo model is physiologically accurate but inefficient due to high cost and time. Since 2D models poorly mimic tumor microenvironment and elicit inaccurate drug response, 3D models such as the tumor spheroid or cells in hydrogel matrix have been considered as advanced platforms [9, 10]. Although ultra-low adhesion (ULA) plates enable 3D cell culture like spheroids and cells in hydrogel matrix, they are limited in creating dynamic culture environment for the cells and continuously replenishing fresh media. Microfluidic systems, where microstructures are precisely arranged, can easily encompass the hydrogel matrix inside the channels, enabling flexible coculture with different cell types and diffusion of nutrients for accurate physiological modeling of cancer with a better control over flow of reagents [10]. Additionally, in microfluidics, researchers have shown to mimic tumor microenvironments with a small volume of biological samples and reagents [11, 12].
There are several types of microfluidic drug screening platforms with varying strengths and weaknesses. These include microdroplet-based chips, microarray-based chips, tumor tissue chips, and concentration gradient generator (CGG)-based chips [10, 13,14,15,16,17,18,19,20]. Microdroplet-based chips minimize contamination and shear stress exposure to the cells, but it is challenging to manipulate the droplets with existing analysis methods [13, 14]. Microarray-based chips can eliminate pipetting steps, but they require complex fabrication processes, equipment for liquid handling, and specialists for implementation [15, 16]. Tumor tissue chips are great for modeling organ-like mechanical environments but are not suitable for building complex structures needed in in vivo on chips [10]. CGG chips are low cost, compatible with high-resolution imaging systems, and connectable to a syringe pump to generate well-defined concentration gradients [17, 18]. They also enable relatively high-throughput screening since cell chambers can be laid out in a series [19]. However, these concentration gradient generators have weaknesses in that they require external instrument like pump for a full set-up and mixing of the drug dilutions depend on diffusion, which can be time-consuming. However, for a drug screening platform with cells exposed to a continuous flow of drug dilutions for multiple days, slow diffusion-based gradient generation is of minor importance. Widely known concentration gradient generators (CGGs) such as Christmas tree mixer networks, T-junction, Y-junction, flow splitters, and pressure balance cover only limited order of gradients by being able to produce either linear or exponential gradients in a single setting [20]. The most well-known type, Christmas tree mixer, is great for generating multiple exponential gradient levels through serial dilution at each node. However, the network of Christmas tree mixers takes up space in a microfluidic device, influencing the overall size of the device to increase. T-junction, Y-junction, and flow splitter types are limited in the range of gradients generation and have constrained microchannel geometries. Pressure balance type is useful for studies that want to minimize non-native shear stress introduced to the cells, but it takes longer to generate concentration gradients. These existing CGGs can generate only linear or logarithmic gradients in a single setting, and it is difficult to maintain stable gradients for multiple days [21, 22].
Here, we develop a simple, inexpensive CGG-based platform that can generate wide (0–100% range) and accurate (R2 = 0.96) concentration gradients of drugs for a high-resolution analysis. Stepwise concentration gradient generator channels were used to simultaneously generate linear and logarithmic gradients [23]. The working design of a stepwise concentration gradient generator is simple in that the ladder-type channels, which were modeled after parallel features in hydraulics and electric circuits, create various concentrations based on different resistance values and drain in their respective cell culture chambers under a continuous flow. For physiological modeling of cancer, 3D hydrogel matrix and cancer cells were embedded in each cell culture chamber. Since bladder cancer is known for high rates of relapse and is limited to cisplatin-centered combination chemotherapy, human urinary bladder cancer cell line (T24) was used on the proposed device [24]. For the proof-of-concept of the multi-layered 3D cell culture incorporated CGG-based drug screening device, T24 cells-embedded-fibrin gel was seeded in the individual cell chambers to model bladder cancer and was exposed to two different chemotherapy drugs separately for the first condition, then two chemotherapy drugs simultaneously for the second condition under the target dilutions. Our simple multi-layered platform could facilitate the drug screening process by extracting optimal dosage for single drug testing and combinatory two drugs testing, which can aid in the best clinical outcome and quality of life for cancer patients.
2 Results and Discussion
2.1 Design of Multi-layer Microfluidic Device with Concentration Gradient and Cell Culture Chamber
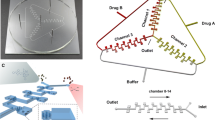
The proposed multilayered microfluidic device for drug screening consisted of two PDMS layers (gradient layer and cell layer) and a glass slide (Fig. 1A, B). The two layers combined allow for integration of a concentration testing platform with cancer cells in the 3D microenvironment to be used as a cancer drug screening platform. The gradient layer generates drug concentration gradients with four 1 mm-diameter inlets and a 4 mm-diameter clearance hole in the center for the waste outlet; two inlets on the left half are for reagent 1, and two inlets on the right half are for reagent 2 (Fig. 1A). To come up with necessary channel dimensions for the designated target concentration levels, which include linear (50%, 20%, 5% 2% drug level) and logarithmic (100%, 10%, 1% drug level) gradients, the following steps and equations were utilized. Working inversely from the target concentration value, necessary flow rate ratio between two reagents (e.g. drug and diluting agent) was calculated. Then, the Hagen–Poiseuille flow equation (Eq. 1) analogous to the Ohm’s law was applied to derive the necessary resistance ratio, which is inversely related to flow rate ratio,
where P is pressure drop, Q is volumetric flow rate, and R is hydraulic resistance [24]. To derive necessary resistance values for the target concentration levels, the following equation was used,
where \({C}_{k}\) is concentration level at chamber k, \({Q}_{{k}_{r1}}\) is flow rate of reagent 1 at chamber k, and \({Q}_{{k}_{r2}}\) is flow rate of reagent 2 at chamber k. For each chamber, the concentration level depends on the volumetric flow rates of reagent 1 and reagent 2 because it is a ratio between the volumetric flow rate of reagent 1 and the total volumetric flow rate at each chamber (Eq. 2). To achieve the desired gradient levels, the flow rate was set to be identical for both inlets of reagent 1 and reagent 2. Resistance equation for rectangular cross-section (Eq. 3) was used to calculate width of the channels leading to each draining port,
where L is channel length, h is channel height, w is channel width, and η is viscosity [10, 25]. The calculated channel widths needed for each chamber is shown in the supplementary information (Fig. S1). To prevent irregular concentration gradients at the onset of infusing the channels, we pre-wetted the microchannels with media to fill up the channels before starting the experiment to ensure uniform surface energy on PDMS channel surface.
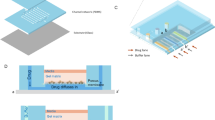
Design and optimization of the proposed multi-layered concentration gradient generator-cell culturing chambers device. A Top view of each layer and assembly are shown here. The top PDMS layer is the gradient layer, consisting of four inlets, draining ports, and a clearance hole for the outlet. The bottom PDMS layer is the cell layer, consisting of the outlet and individual cell culturing chambers. Detail features of the cell culturing chamber is shown in the top right corner. The exploded view of the overall chip is shown in the second row. B Real image of the multi-layered microfluidic device filled with red dye in its channels is shown with the penny for size comparison. C Schematic of the experimental set-up. The device is connected to a syringe pump that is infusing reagent 1 and reagent 2 at 0.2 mL/h flow rate and a waste reservoir. For assays, the device is subsequently placed under the inverted fluorescent microscope to be imaged
In the electric circuit diagram, each draining port for an individual cell culturing chamber, which is equidistant to the outlet, and the four inlets on the gradient layer were modeled as ground since they all have atmospheric pressures. Because microchannels were connected in both series and parallel, an interactive circuit simulator was used to facilitate calculations on the flow rates of the respective resistance from the modulated channel width. A free interactive circuit simulator software from Lush Project (General Public License as published by Free Software Foundation, https://lushprojects.com/circuitjs/) was used to modulate channel width values for multiple channels and see resulting flow rates to match the target concentration levels (Fig. S2).
The cell layer has circular cell culturing chambers (CCCs), which encompass uniformly spaced out trapezoidal posts that hold the fibrin gel-embedded-T24 cells in place under surface tension (Fig. 1A). Mixing channels from the draining port to the CCC region were adequate for delivering intended drug gradient level to the 3D-cell culture interfaces at 0.2 mL/h infusion flow rate (Fig. 3C). The cell layer has a 1 mm-diameter-sized outlet in the center and 10 independent chambers that are equally spaced out in a 9 mm interval laterally (standardized adjacent well spacing in 96-well plate) to be compatible with a microplate reader to make this device accessible for variety of analysis in the future (Fig. 1A, Fig. S3). The gasket for placing the microfluidic chip inside the plate reader was designed using SolidWorks (Dassault Systèmes) and 3D-printed in a polylactic (PLA) material as shown in Figure S3.
The shape of the cell culturing region is optimized, considering the rate of diffusion is higher with greater contact surface area. To facilitate transport of the cell media and drug to the CCC, the region was designed to be circular in shape. To further enhance diffusion, the drug-media channel was designed to be symmetrical around the circular cell culturing area. Given the design constraint of 9 mm spacing between CCCs for plate reader compatibility, efficient mixing within the confined space is critical. Mixing channels need an appropriate mixing length for inducing a high mixing efficiency; following equations (Eqs. 4, 5) were used with estimation from Fick’s Law,
where Pe is Peclet number, l is characteristic length, U is velocity, D is mass diffusion coefficient, \({l}_{{\text{mix}}}\) is mixing channel length, and \({t}_{{\text{mix}}}\) is time needed for mixing [26,27,28]. Using 0.2 mL/h flow rate and 1990 μm2/s mass diffusion coefficient (for cisplatin in water at 27 °C), Pe was calculated to be 0.44, and the minimal mixing channel length was calculated to be 0.022 mm [29]. To accommodate higher flow rate and greater diffusion coefficient, which is dependent on solute’s molecular weight, property of the solvent, and temperature, the sinusoidal design was selected for the mixing channels [29, 30]. Sinusoidal mixing channel for each cell chamber was designed to be 7 mm long, and the time needed for mixing in these channels was calculated to be 2.5 s given 0.2 mL/h flow rate using Eq. 5. Dimensions and spacings of the trapezoidal posts in the CCCs were optimized to robustly hold hydrogel under surface tension.
2.2 Biocompatibility of the Stepwise CGG-CCC Microfluidic Device
A double-layered microfluidic chip with a stepwise concentration gradient generator and individual cell culturing chambers was designed to enable simultaneous generation of the linear and logarithmic gradients for screening anti-cancer drugs (Fig. 2A). Prior to the cell experiments with chemotherapy drugs (cisplatin and gemcitabine), the proposed microfluidic device was assessed for its biocompatibility by running cell culturing media through the four inlets. Depending on the key characteristics to be modeled on a specific tumor-on-chip, previous studies have characterized various strengths and weaknesses of commonly used hydrogel materials [31, 32]. Since T24 is an invasive transitional cell carcinoma and forms transitional epithelium consisting of several layers of cells that stretch and line the bladder, it is appropriate to model T24 cells in a 3D hydrogel matrix [33]. Among various types of hydrogel, fibrin gel was selected for this study because its nano- and micro- architecture mimics the native extracellular matrix accurately. Fibrin gel also allows for a wide range of stiffness, which is essential for bladder lining frequently stretching and contracting [31]. As a result, fibrin gel was selected for the 3D cell culture of T24 cells in hydrogel matrix for this study [34, 35].
Cell viability of the cell layer with independent 3D cell culturing chambers. A Schematic of the multi-layered CGG–CCC device is shown. Below, a cross section of a cell culturing chamber is labelled, where the central circular region has T24 cells in fibrin gel under surface tension surrounded by trapezoidal posts, and the drug–media channel wraps around the circular cell chamber. B Representative fluorescent image of a cell culturing chamber (post-live dead assay) with T24 cells embedded in fibrin gel is shown here, where green-fluorescent cells are live cells, and red-fluorescent cells are dead cells. The scale bar is 200 μm. C Percentage of live cells in each chamber was quantified and plotted. Total of five chips (n = 5 independent chips) were tested for the cell viability experiment with chips exposed to 24 h of media only at a continuous flow of 0.2 mL/h. The error bar is standard error. P value from one-way ANOVA test was 0.92, confirming at 0.05 level, the means are not significantly different across the chambers
Chips seeded with T24 cells and fibrin gel were connected to a syringe pump for continuous infusion of the cell culturing media for 24 h. At the end of the 24 h of continuous infusion, the individual CCCs were assessed with a live/dead assay. Each cell chamber was imaged under the microscope and processed to assess cell viability.
Although there are 10 cell chambers on the device layout, only 8 chambers were used in our study because the 2 remaining chambers served as a buffer to help create designated target concentration levels in other chambers. To assess biocompatibility of our device, all cell chambers were under 24 h continuous infusion of the cell culturing media without drug, and high cell viability was observed for all 8 chambers, where 77–86% cells were kept alive (Fig. 2B, C). One-way ANOVA test was done with a resulting P value of 0.92; at 0.05 level, cell viability across all chambers are consistent and not significantly different (Fig. 2C).
2.3 Target Gradient Validation with On-Chip Fluorescence Measurements
To validate linear and logarithmic concentration levels generated from the gradient layer of the multi-layered microfluidic platform, a fluorescent image of the channel corresponding to each chamber was processed by averaging out the fluorescent intensity values at 3 regions marked in yellow boxes (Fig. 3A). All fluorescence intensity values were normalized against the chamber 1 (100% drug level)’s fluorescence intensity value. Experimental fluorescence intensity values were obtained from 4 devices, and the normalized values were plotted against the target theoretical values (Fig. 3B). The extracted R2 value is 0.96, showing a high correlation between the theoretical values and the experimental values (Fig. 3C). The gradient level validation data demonstrate consistency with the target values (100%, 50%, 20%, 10%, 5%, 2%, 1%, 0%) across each chamber, with slightly higher experimental values than the target. Numerical results of the theoretical and experimental values at each chamber are shown in Fig. S4. Slight discrepancy between the experimental and theoretical values across the chambers may result from fabrication error of misalignment that could arise from manually punching out each of the draining ports and from the bonding process, where the gradient layer and cell layer could be misaligned since the steps are done manually. It is interesting to note that the experimental fluorescence intensity for chamber 5 is marginally higher than that of chamber 4, most likely due to its proximity to the drug inlet. The overall resulting concentration levels confirm the simultaneous generation of both logarithmic and linear gradient levels.
On-chip validation of linear and logarithmic gradient levels. A The reagents channel of each chamber was imaged four hours after continuous infusion of fluorescein and distilled water. Fluorescent images of each chamber are shown. Three yellow boxes indicate the region of interest where the fluorescent intensity values were extracted. The scale bar is 600 μm. Fluorescent images were adjusted for enhanced visualization. B Comparison of experimental fluorescence intensity values (in red) from four chips (n = 4 independent chips) and the target theoretical values (in blue), where the error bar is standard error = σ/\(\sqrt{n}\). The target theoretical values are derived from the electric circuit analogy calculations. Raw data of this plot can be found in Figure S6 in the supplementary material. C Experimental fluorescence intensity values (Y-axis) from the same four chips (n = 4 independent chips) are plotted against the respective theoretical values (X-axis), where R2 = 0.96. The target theoretical values are derived from the electric circuit analogy calculations
A slight discrepancy between the theoretical value and experimental value in the individual chamber may be due to several limitations in addition to fabrication error. Theoretical concentration value at each chamber was calculated from the electric circuit model, which does not consider diffusion properties of individual reagents. It may contribute to the discrepancy because the electric schematic diagram used for modeling gradient levels did not specifically consider the effect of diffusion induced by differing molecular weight of reagents and assumed uniform molecular weight of all reagents [36]. There exists a design limitation where due to the proximity of chamber 5 being located near the drug inlet and chamber 4 being located near the diluting reagent inlet, chamber 5 had marginally higher fluorescence level. However, since R2 value is 0.96, the experimental values can be considered in line with the target values (Fig. 3C). Thus, theoretical gradient values were verified against the experimental values via on-chip fluorescence intensity measuring experiments.
2.4 Evaluation of Cisplatin Efficacy on T24 Cells
Live/dead assay results from devices with T24 cells, after 48 h exposure to cisplatin and diluting media, were processed (Fig. 4). Cisplatin was used for the single drug experiment against T24 cells because cisplatin is the primary chemotherapy drug used to treat muscle-invasive bladder cancer [34]. Fluorescent images of three distinct drug level chambers following the live/dead assay are shown, where chamber 1 is 100% drug level (66.42 μM cisplatin), chamber 2 is 50% drug level (41.76 μM cisplatin), and chamber 8 is 0% drug level (0.58 μM cisplatin). As shown on the drug dose response curve, processing eight cell chambers in five chips yielded these cell viability rates at respective drug doses (Fig. 4). Experimental concentration values from Fig. 3B were used for plotted log-drug concentration values in the drug dose–response curve for all subsequent figures to address slight discrepancy between theoretical and experimental concentration values. IC50 is a common parameter used for assessing the potency of anti-cancer drugs, and it is defined as the concentration of drug needed for only 50% of the cancer cells to be alive. From this study, the IC50 value of cisplatin-induced apoptosis on the 3D-cultured T24 cells embedded in fibrin gel was 7.11 μM, which is similar to IC50 value 9.03 μM of T24 cell-spheroids reported in Wu et al. [36]. It is interesting to note that a 2D culture of T24 cells previously resulted in different IC50 values, 3.52 μM and 4.12–4.98 μM, from 3D culture [10, 37]. Extracted IC50 values from these recent 3D culture studies provide new insights into drug dose response.
On-chip cisplatin-induced apoptosis analysis (single drug screening). Live/dead assay of chips with T24 cells after 48 h of exposure to cisplatin and RPMI at a continuous flow of 0.2 mL/h. Drug dose–response curve of cisplatin-induced apoptosis of five devices (n = 5 independent chips) is plotted, where the extracted IC50 is 7.11 μM. The error bar is standard error = σ/\(\sqrt{n}\). Raw data of this plot can be found in Figure S7 in the supplementary material. Fluorescent images of 0.58 μM cisplatin, 41.76 μM cisplatin, and 66.42 μM cisplatin drug chambers are shown, where green-fluorescent (calcein-AM) cells are live cells, and red-fluorescent (ethidium homodimer-1) cells are dead cells. The scale bar is 400 μm
To further validate cell experimental data from the live/dead assay, the proliferation assay was conducted after 48 h of exposure to cisplatin and cell media (Fig. S5). The ratio between EdU positive proliferative cells to counterstained DAPI positive cells was plotted against the cisplatin dose in line with the live/dead assay. Representative fluorescent images of Click-iT EdU and DAPI stained cells are shown, where all proliferating cells are stained in red, and all cell nuclei are stained in blue (Fig. S5). Fluorescent images of three distinct drug level chambers following the proliferation assay are shown. Proliferation rate analysis of six devices revealed a positive correlation between percentage of proliferating cells and decreasing drug dosage. The overall trend of proliferation rates from this study, 14% at 6.64 μM cisplatin and 34% at 0.58 μM cisplatin, mirrors previously reported trend of proliferation rates, 3% at 6.64 μM cisplatin and 20% at 0 μM cisplatin [38]. Numerical differences could be attributed to different experimental designs such as using different cell culturing media.
2.5 Evaluation of Gemcitabine Efficacy on T24 Cells
In addition to cisplatin, gemcitabine was selected for single drug screening on this platform. Since gemcitabine is another standard chemotherapy regimen with cisplatin for treating muscle-invasive bladder cancer, we evaluated live/dead assay results after exposing T24 cells for 48 h with gemcitabine [39]. Fluorescent images of three distinct drug level chambers following the live/dead assay are shown, where chamber 1 is 100% drug level (100 μM gemcitabine), chamber 2 is 50% drug level (62.87 μM gemcitabine), and chamber 8 is 0% drug level (0.87 μM gemcitabine). Processing 8 cell chambers in three chips yielded the following cell viability rates at respective drug doses (Fig. 5). Plotted log-drug concentration values, in the drug dose–response curve, reflect experimental values from Fig. 3B. From this study, the IC50 value of gemcitabine-induced apoptosis on the 3D-cultured T24 cells embedded in fibrin gel was 12.94 μM.
On-chip comparison of gemcitabine-induced apoptosis and gemcitabine-cisplatin-induced apoptosis. Live/dead assay of chips with T24 cells after 48 h of exposure to gemcitabine in RPMI or gemcitabine and cisplatin in RPMI at a continuous flow of 0.2 mL/h. Drug dose–response curves of gemcitabine-induced apoptosis of three devices (n = 3 independent chips) and gemcitabine + cisplatin-induced apoptosis of three devices (n = 3 independent chips) are plotted, where the extracted IC50 for gemcitabine only (orange curve) is 12.94 μM and the extracted IC50 for gemcitabine at fixed cisplatin dose (7.11 μM) (purple curve) is 9.44 μM. The error bar is standard error = σ/\(\sqrt{n}\). Raw data of this plot can be found in Figure S8 and Figure S9 in the supplementary material. Fluorescent images at designated drug level chambers are shown, where green-fluorescent (calcein-AM) cells are live cells, and red-fluorescent (ethidium homodimer-1) cells are dead cells. The scale bar is 400 μm
2.6 Evaluation of Gemcitabine-Cisplatin Combined Efficacy on T24 Cells
Finally, combination of two drugs, cisplatin and gemcitabine, was tested to investigate whether they have synergistic or antagonistic efficacy. Previously, multi-drug therapy has been shown to enhance therapeutic effects of drugs for T24 cells in 2D platforms [40, 41]. We fixed the concentration level of cisplatin to its IC50 value from the single drug experiment while testing the varying concentration range of gemcitabine to see the effects of combination therapy. Similar to single drug experiments, three devices with T24 cells in fibrin gel were processed after 48 h exposure to varying doses of gemcitabine and fixed 7.11 μM cisplatin concentration in diluting cell media (Fig. 5). Fluorescent images of the three representative combination drug levels are presented from top to bottom.
Similar to the single drug experiment, sigmoidal drug dose response curve was used to demonstrate the combinatorial drug screening results and facilitate comparison of the different drug conditions in this study, instead of popular heat maps with Bliss score and combination index, due to limited data points in the variation of combinatory drug doses. The plotted log-drug concentration values for gemcitabine, in the drug dose–response curve, reflect experimental values from Fig. 3B. It is interesting to note the differences in IC50 values for each drug condition. For single drug exposure conditions, the IC50 values were 7.11 μM for cisplatin only and 12.94 μM for gemcitabine only. For combinatory exposure condition to fixed cisplatin dose and gemcitabine, the value was 9.44 μM. Approximately, 27% decrease in the IC50 value for gemcitabine in the combinatory exposure condition, compared to the single drug condition, is likely due to the different working mechanisms of two chemotherapy drugs prominently used for treating bladder cancer. While cisplatin is an alkylating agent that damages DNA of rapidly dividing cells by forming platinum–DNA adducts and elicits apoptosis, gemcitabine is an antimetabolite that inhibits DNA polymerase from synthesizing new DNA strands of rapidly dividing cells [40]. Since all cell chambers were exposed to a fixed IC50 value of cisplatin (7.11 μM), the minimum cell death rate in combinatory setting was 60.95 ± 11.80%. Resulting cell death rates confirm enhanced efficacy of gemcitabine on inducing apoptosis in the T24 cells when used in combination with cisplatin. Although our combinatory result is preliminary and uses one fixed cisplatin value, this platform can be expanded to screen with different cisplatin values against gemcitabine gradient levels. Thus, our study could expedite implementing optimal drug dose for gemcitabine-cisplatin combinatory chemotherapy regimen, a commonly known strategy for treating bladder cancer, using the multi-drug screening platform to extract optimal drug doses for two drugs combination. Furthermore, by mix-and-matching two different treatment strategies, this platform could be further utilized for screening combined or targeted therapy of hydrophilic drugs for cancer treatments.
This study adds value to accelerating the inefficient process of drug screening because our system is able to generate both linear and logarithmic concentration gradients simultaneously and encompasses individual cell culturing chambers for 3D cell culture in a dynamic culture setting; however, there still remains future work for improving this study. For better control of the batch-to-batch variability of the cell densities seeded in each cell chamber, 3D bio-printing technology could be used in the future studies to prevent the differences that can arise from manual pipetting [42]. Since 3D bio-printing technology creates structured and uniform tissues of various cell lines, it could potentially replace the 3D cell cultures being manually seeded into our device. It could also be interesting to vary the duration of drug exposure from 48 to 24 h and 72 h to observe how drug exposure time affects anti-cancer drug induced apoptosis of the cancer cells. Since this device is simple in design, it could pave a road for ongoing innovative studies involving different types of cell culturing layers with co-culture of cells for modeling more complex biological microenvironment given the innate limitations of lab-on-chip. This device could be used to investigate apoptosis of other types of cancer cells like glioblastoma, with more physiological relevance in the 3D culture, or for antibiotic susceptibility testing by seeding bacteria in the cell culturing layer.
3 Conclusion
Diversifying the drug dosage range and shifting towards 3D cell culture from 2D cell culture for drug screening are crucial for optimizing the highly inefficient drug discovery and development process. In this study, we demonstrated a multi-layered microfluidic device that can simultaneously generate linear and logarithmic gradient levels and house independent 3D cell culturing chambers for 48 h for drug screening application. The cell layer of the device was designed to match the well spacings of the 96-well plate to be compatible with the standard microplate reader to acquire further readouts for deeper analysis. We generated and maintained eight different gradient levels, consisting of linear and logarithmic concentrations, under the continuous flow rate of 0.2 mL/h using a syringe pump. On this simple microfluidic device, drug-induced apoptosis was evaluated for (1) cisplatin only, (2) gemcitabine only, and (3) gemcitabine and cisplatin on 3D cultured human bladder cancer cells, showing the advantage of using combination chemotherapy over monotherapy for treating muscle-invasive human bladder cancer. We believe that this simple multi-layered device could facilitate implementation of microfluidics in drug evaluations and combinatory therapy screenings for treating cancer and other diseases.
4 Materials and Methods
4.1 Fabrication of Multi-layered Microfluidic Platform
Standard soft-photolithography protocols were used to make SU-8 (SU-8 2050, MicroChem) Si wafer master molds for the gradient layer and cell layer with 50 μm microchannel height [43]. At a ratio of 8:1 (base:curing agent), polydimethylsiloxane (PDMS, Sylgard 184, Dow Corning) was prepared to be poured over master molds and degassed with a vacuum desiccator. After curing the PDMS layers in a 60 °C oven, they were carved out with a surgical blade. Biopsy punches (KAI Medical) of diameter size 1 mm, 1.5 mm, 2 mm, and 4 mm were used to carve out inlets, draining ports, and outlets. Carved layers were placed in 70% (v/v) ethyl alcohol inside the sonicator for 10 min for sterilization and dried in a 60 °C oven. The sterilized layers were treated with oxygen plasma (CUTE, Femto Science) for the bonding process. Bonded PDMS layers was then bonded to a sterilized glass slide of 1.5 mm thickness (Matsunami) with oxygen plasma treatment. The assembled device was stored in a 60 °C oven for 12 h before validation experiment and cell experiment for recovering hydrophobic properties of the PDMS layers.
4.2 Characterization of the Concentration Gradient Generator (CGG)
To validate concentration levels generated from the gradient layer, 10 µg/mL fluorescein dye diluted in distilled water (reagent 1 in Fig. 1C) and distilled water (reagent 2 in Fig. 1C) were continuously infused into the device at a volumetric flow rate of 0.2 mL/h for 4 h by a syringe pump (Harvard Apparatus) to fill up all reagent channels of gradient layer and cell layer and imaged under an inverted fluorescent microscope (Axio Observer Z1, Zeiss). Fluorescein dye was used because it has a molecular weight 332.31 g/mol, which is close to the molecular weight of cisplatin 301.1 g/mol. The drug-media channel of each chamber of the cell layer was imaged to measure the fluorescence intensity. Background intensity values of each image was subtracted from the original image to accurately extract the experimental fluorescence intensity values. ImageJ-ROI Manager was used to extract the average fluorescence intensity value at three yellow boxes labelled at each chamber (Fig. 3A). Extracted values were compared with theoretical values (Fig. 3B, C)).
4.3 Cell Culture
T24 cell, a human bladder cancer cell line, was obtained from American Type Culture Collection (Rockville, MD, USA). T24 cells were cultured in RPMI-1640 medium (Gibco) supplemented with 10% fetal bovine serum (FBS, Gibco) and 1% penicillin–streptomycin (PS, Gibco). Cells were maintained in a humidified incubator with 37 °C and 5% CO2.
4.4 Cell Seeding and Experimental Setup
T24 cells were seeded with fibrin gel in the device for a 3D cell culture (Fig. 2A, B). To prepare fibrin gel, fibrinogen powder (Sigma-Aldrich) was diluted in phosphate-buffered saline (PBS) to make 6 mg/mL fibrinogen solution. The fibrinogen solution was mixed with 4 U/mL thrombin solution (Sigma-Aldrich) at a 1:1 (v/v) ratio to make 3 mg/mL fibrin gel. Resulting fibrin gel and T24 cells were mixed at a 1:1 (v/v) ratio, and the mixture was immediately seeded into the chip through the gel inlet of the cell layer (Figs. 1A, 2A). The seeding density of the T24 cells was 4 × 105 cells/mL and the final concentration of the fibrin gel was 1.5 mg/mL.
After a 30–40 min gelation period of the chip in a humid chamber inside the incubator (37 °C, 5% CO2), reagent channels of the chip were filled with RPMI. The seeded chip was connected to a syringe pump for continuous infusion of two reagents for 48 h (Fig. 1C). Then, cell viability or proliferation status was assayed subsequently.
4.5 Preparation of Anti-cancer Drugs
For the first condition of the single drug screening experiment, 100% drug level in the chip was set at 66.42 μM cisplatin. The cisplatin stock (Dong-A ST) was diluted in cell culturing media to prepare a 66.42 μM cisplatin solution. To evaluate cytotoxicity of cisplatin on the 3D hydrogel-embedded bladder cancer cells, cisplatin and cell medium were continuously infused to the chip at a volumetric flow rate of 0.2 mL/h for 48 h and subsequently assayed. For the second condition of the single drug screening experiment, gemcitabine was used. For 100% drug level in the chip, gemcitabine concentration was fixed at 100 μM. Gemcitabine solution was prepared by dissolving gemcitabine powder (Laurus Labs) in PBS and then diluting the stock gemcitabine solution in cell culturing media to create 100 μM gemcitabine solution. To evaluate cytotoxicity of gemcitabine on the 3D cultured bladder cancer cells, gemcitabine and cell medium were continuously infused to the chip at a volumetric flow rate of 0.2 mL/h for 48 h and subsequently assayed.
For the combinatorial drug screening, each cell chamber with T24 cells was exposed to different doses of gemcitabine. For 100% drug level, gemcitabine concentration was set at 100 μM and fixed cisplatin dosage of 7.11 μM, which is the IC50 value. Gemcitabine solution was prepared by dissolving gemcitabine powder in PBS. Reagent 1 was prepared by diluting stock gemcitabine solution in 7.11 μM cisplatin-RPMI solution to create 100 μM gemcitabine reagent. Reagent 2 was prepared by diluting cisplatin stock in cell culturing media to create a 7.11 μM. These two reagents were continuously infused to the chip at a volumetric flow rate of 0.2 mL/h for 48 h.
4.6 Live/Dead Assay
A live/dead assay kit (Invitrogen), with calcein-AM and ethidium homodimer-1 components, was conducted for biocompatibility testing at the end of 48 h exposure of the drug dilutions to quantify cisplatin-induced apoptosis on the cells. After 2 days of drug exposure, the cells were stained in 10 mL PBS solution with 5 μL calcein-AM and 20 μL ethidium homodimer-1 and incubated for 1 h. The cells inside the microfluidic device were imaged under an inverted fluorescent microscope. Specifically, all cells inside the white dotted boundary region were counted for the live cells percentage calculation per chamber (Fig. 2B, C). Fluorescent images of each cell chamber were processed by ImageJ software using the Watershed Algorithm. Cell viability was calculated by dividing the total number of live cells (calcein-AM stained) by the total number of cells (both calcein-AM and ethidium homodimer-1 stained).
4.7 Proliferation Assay
To cross-validate the drug-induced cytotoxicity results from live/dead assay, proliferation assay was conducted on the cisplatin only experimental chips. DNA synthesis-based proliferation assay called Click-iT Ethynyl deoxyuridine (EdU) Imaging Kit (Invitrogen) was used, and instructions were followed as stated in the manufacturer’s manual. This proliferation assay works when the EdU reagents, which are the modified thymidine analogs, get tagged onto a newly synthesized DNA, which in turn becomes labeled with a fluorescent photostable Alexa Fluor dye via click reaction. To measure the proliferation rate, 4′,6-diamidino-2-phenylindole (DAPI, Invitrogen) was used to stain the nuclei of all seeded cells to count the total number of cells in each chamber. Subsequently, each cell chamber image was processed by ImageJ software using the Watershed Algorithm. The cell proliferation rate was calculated by dividing the number of EdU-positive cells by the total number of cells (DAPI stained).
4.8 Statistical Analyses
All data points are shown with ± standard error of the mean. Data plotting, drug dose–response curve fitting (sigmoidal function in nonlinear fitting), one-way ANOVA, and analyses were all performed on Origin 2019 Software (OriginLab). IC50 values were extracted from the dose–response curves.
Data availability
The data is available on request to the corresponding author.
References
DeVita, V.T., Rosenberg, S.A., Engl, N.: J. Med. 366(23), 2207–2214 (2012)
Centers for Disease Control and Prevention. https://www.cdc.gov/nchs/nvss/deaths.htm?CDC_AA_refVal=https%3A%2F%2Fwww.cdc.gov%2Fnchs%2Fdeaths.htm. Accessed July 2022.
Boyle, P., Levin, B.: World Cancer Report 2008. IARC Press, Lyon (2008)
Senapati, S., Mahanta, A.K., Kumar, S., Maiti, P.: Signal Transduct. Target. Ther. 7, 3 (2018)
Waldman, A.D., Fritz, J.M., Lenardo, M.J.: Nat. Rev. Immunol. 20(11), 651–668 (2020)
Larsson, P., Engqvist, H., Biermann, J., Werner Rönnerman, E., Forssell-Aronsson, E., Kovács, A., Karlsson, P., Helou, K., Parris, T.Z.: Sci. Rep. 10(1), 5798 (2020)
Mokhtari, R.B., Homayouni, T.S., Baluch, N., Morgatskaya, E., Kumar, S., Das, B., Yeger, H.: Oncotarget 8(23), 38022–38043 (2017)
Lee, D.W., Choi, Y.-S., Seo, Y.J., Lee, M.-Y., Jeon, S.Y., Ku, B., Kim, S., Yi, S.H., Nam, D.-H.: Anal. Chem. 86(1), 535–542 (2014)
Boussommier-Calleja, A., Li, R., Chen, M.B., Wong, S.C., Kamm, R.D.: Trends Cancer 2(1), 6–19 (2016)
Wang, X., Liu, Z., Pang, Y.: RSC Adv. 7, 29966–29984 (2017)
Bae, J., Kim, M.-H., Han, S., Park, S.: BioChip J. 17, 77 (2022)
Park, S.Y., Hong, H.J., Lee, H.J.: BioChip J. 17, 24 (2022)
Boedicker, J.Q., Li, L., Kline, T.R., Ismagilov, R.F.: Lab Chip 8, 1265–1272 (2008)
Iino, R., Hayama, K., Amezawa, H., Sakakihara, S., Kim, S.H., Matsumono, Y., Nishino, K., Yamaguchi, A., Noji, H.: Lab Chip 12, 3923–3929 (2012)
Ding, Y., Li, J., Xiao, W., Xiao, K., Lee, J., Bhardwaj, U., Zhu, Z., Digiglio, P., Yang, G., Lam, K.S., Pan, T.: Anal. Chem. 87(20), 10166–10171 (2015)
Hvastkovs, E.G., Rusling, J.F.: Anal. Chem. 88(9), 4584–4599 (2016)
Jeon, N.L., Dertinger, S.K., Chiu, D.T., Choi, I.S., Stroock, A.D., Whitesides, G.M.: Langmuir 16(22), 8311–8316 (2000)
Montón, H., Medina-Sánchez, M., Soler, J.A., Chałupniak, A., Nogués, C., Merkoçi, A.: Biosens. Bioelectron. 94, 408–414 (2017)
Fei, J., Wu, L., Zhang, Y., Zong, S., Wang, Z., Cui, Y.: ACS Sens. 2(6), 773–780 (2017)
Toh, A.G., Wang, Z.P., Yang, C., Nguyen, N.-T.: Microfluid. Nanofluid. 16, 1–18 (2014)
Ye, N., Qin, J., Shi, W., Liu, X., Lin, B.: Lab Chip 7, 1696–1704 (2007)
Tang, M., Huang, X., Chu, Q., Ning, X., Wang, Y., Kong, S.-K., Zhang, X., Wang, G., Ho, H.-P.: Lab Chip 18, 1452–1460 (2018)
Weibull, E., Matsui, S., Sakai, M., Andersson Svahn, H., Ohashi, T.: Biomicrofluidics 7(6), 064115 (2013)
Liu, Q., Zhang, C., Ding, X., Deng, H., Zhang, D., Cui, W., Xu, H., Wang, Y., Xu, W., Lv, L., Zhang, H., He, Y., Wu, Q., Szyf, M., Ho, C.-M., Zhu, J.: Sci. Rep. 5, 11464 (2015)
Kim, T., Jo, K.: BioChip J. 17, 384 (2023)
Friedrich, D., Please, C.P., Melvin, T.: Chem. Eng. J. 193–194, 296–303 (2012)
Rismanian, M., Saidi, M.S., Kashaninejad, N.: Chem. Eng. Sci. 195, 120–126 (2019)
Chen, X., Li, T., Zeng, H., Hu, Z., Fu, B.: Int. J. Heat Mass Transf. 98, 131–140 (2016)
Nejad, M.A., Urbassek, H.M.: Biomolecules 9(5), 204 (2019)
Chan, T.C., Li, H.T., Li, K.Y.: J. Phys. Chem. B 119(51), 15718–15728 (2015)
Dhiman, N., Kingshott, P., Sumer, H., Sharma, C.S., Rath, S.N.: Biosens. Bioelectron. 137, 236–254 (2019)
Lee, J.-H., Kim, S.-K., Khawar, I.A., Jeong, S.-Y., Chung, S., Kuh, H.-J.: J. Exp. Clin. Cancer Res. 37(1), 4 (2018)
Cleveland Clinic. https://my.clevelandclinic.org/health/articles/22062-epithelium. Accessed Aug 2022.
Teo, M.Y., Rosenberg, J.E.: Nat. Rev. Urol. 16, 75–76 (2019)
Wu, K.W., Kuo, C.T., Tu, T.Y.: Global Chall. 5, 2000056 (2020)
Oh, K.W., Lee, K., Ahn, B., Furlani, E.P.: Lab Chip 12, 515–545 (2012)
Hong, J.H., Lee, E., Hong, J., Shin, Y.J., Ahn, H.: BJU Int. 90(1), 113–117 (2002)
Kim, S., Park, J., Ho, J., Kim, D., Lee, S., Jeon, J.S.: Biofabrication 15(4), 045016 (2023)
Kim, L., Vahey, M.D., Lee, H.-Y., Voldman, J.: Lab Chip 6, 394–406 (2006)
Ma, Y., Yu, W.-D., Trump, D.L., Johnson, C.S.: Cancer 116(13), 3294–3303 (2010)
Mey, V., Giovannetti, E., Braud, F.D., Nannizzi, S., Curigliano, G., Verweij, F., Cobelli, O.D., Pece, S., Tacca, M.D., Danesi, R.: Br. J. Cancer 95, 289–297 (2006)
Mohammadrezaei, D., Moghimi, N., Vandvajdi, S., Powathil, G., Hamis, S., Kohandel, M.: Sci. Rep. 13, 1211 (2023)
Bhatia, S.N., Ingber, D.E.: Nat. Biotechnol. 32, 760–772 (2014)
Acknowledgements
We thank Prof. Sangchul Lee at Seoul National University Bundang Hospital for generously providing us with human bladder cancer cells (T24 cell line) and anti-cancer drugs. This study was supported by National Research Foundation of Korea (NRF-2020R1A5A8018367, 2020R1A2C1100471) and the Brain Korea 21 Plus project.
Funding
Open Access funding enabled and organized by KAIST.
Author information
Authors and Affiliations
Contributions
Jung Yun S. Yoon: conceptualization (lead); data curation (lead); formal analysis (lead); methodology (lead); validation (lead); visualization (lead); writing—original draft (lead); writing—review and editing (equal). Joonha Park: formal analysis (supporting); writing—review and editing (equal). Hyeono Nam: methodology (supporting); writing—review and editing (equal). Seunggyu Kim: conceptualization (supporting); writing—review and editing (equal). Jessie S. Jeon: conceptualization (supporting); funding acquisition (lead); project administration (lead); resources (lead); supervision (lead); visualization (supporting); writing—review and editing (equal).
Corresponding author
Ethics declarations
Conflict of interest
The authors have no relevant financial or non-financial interests to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
13206_2024_154_MOESM1_ESM.docx
Supplementary file1 See the supplementary material for channel width dimension of the gradient layer (Fig. S1), electric schematic of the gradient layer (Fig. S2), 3D-printed gasket for plate reader (Fig. S3), gradient level validation (Fig. S4), proliferation assay of cisplatin-induced apoptosis (Fig S5), and raw data point plots (Fig. S6-Fig. S9). (DOCX 1939 KB)
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Yoon, J.Y.S., Park, J., Nam, H. et al. Multi-layered Microfluidic Drug Screening Platform Enabling Simultaneous Generation of Linear and Logarithmic Concentration Gradients. BioChip J (2024). https://doi.org/10.1007/s13206-024-00154-2
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13206-024-00154-2