Abstract
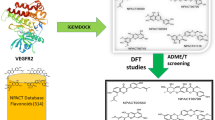
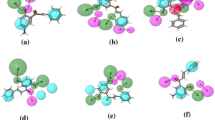
Virtual screening of a library of 329 flavonoids obtained from the NPACT database was performed to find out potential novel HDAC2 inhibitors. Eleven out of 329 selected flavonoids were screened based on molecular docking studies, as they have higher binding affinities than the standard drugs vorinostat and panobinostat. All screened compounds occupying the catalytic site of HDAC2 showed important molecular interaction with Zn2+ and other important amino acids in the binding pocket. The screened compounds were validated using ADMET filtration and bioactivity prediction from which we obtained six compounds, NPACT00270, NPACT00676, NPACT00700, NPACT001008, NPACT001054, and NPACT001407, which were analyzed using DFT studies. DFT studies were performed for all six screened flavonoids. In DFT studies, three flavonoids, NPACT00700, NPACT001008, and NPACT001407, were found to be better based on HOMO–LUMO and molecular electrostatic potential (MEP) analyses. Furthermore, MD simulations were performed for 100 ns for the three compounds. In the MD analysis, NPACT001407 was found to be more stable in the active site of HDAC2 as zinc formed a coordination bond with ASP181, HIS183, ASP269, and GLY305, along with two hydroxyl groups of the ligand. Our findings reveal that these flavonoids can interact as ligands with the active site of HDAC2. Because of the absence of a hydroxamate group in flavonoids, there are no possibilities for the formation of isocyanate. This suggests that the major drawback of current HDACs inhibitors may be solved. Further experimental validation is needed to understand the selectivity of flavonoids as HDAC2 inhibitors.








Similar content being viewed by others
Data availability
The authors declare that the data supporting the findings of this study are available within the paper. Any raw data files needed are available from the corresponding author upon reasonable request.
References
Atahan A, Gencer N, Bilen C, Yavuz E, Genc H, Sonmez F, Zengin M, Ceylan M, Kucukislamoglu M (2018) Synthesis, biological activity and structure-activity relationship of novel diphenylurea derivatives containing tetrahydroquinoline as carbonic anhydrase I and II inhibitors. ChemistrySelect 3(2):529–534. https://doi.org/10.1002/slct.201702562
Banerjee P, Eckert AO, Schrey AK, Preissner R (2018) ProTox-II: a webserver for the prediction of toxicity of chemicals. Nucleic Acids Res 46(W1):W257-w263. https://doi.org/10.1093/nar/gky318
Becke AD (1993) A new mixing of Hartree–Fock and local density-functional theories. J Chem Phys 98(2):1372–1377. https://doi.org/10.1063/1.464304
BIOVIA DS (2021) Discovery studio 2021 client, San Diego: Dassault Systèmes. Discovery studio
Bondarev AD, Attwood MM, Jonsson J, Chubarev VN, Tarasov VV, Schiöth HB (2021) Recent developments of HDAC inhibitors: emerging indications and novel molecules. Br J Clin Pharmacol 87(12):4577–4597. https://doi.org/10.1111/bcp.14889
Bowman GD, Poirier MG (2015) Post-translational modifications of histones that influence nucleosome dynamics. Chem Rev 115(6):2274–2295. https://doi.org/10.1021/cr500350x
Bressi JC, Jennings AJ, Skene R, Wu Y, Melkus R, Jong RD, O’Connell S, Grimshaw CE, Navre M, Gangloff AR (2010) Exploration of the HDAC2 foot pocket: synthesis and SAR of substituted N-(2-aminophenyl)benzamides. Bioorg Med Chem Lett 20(10):3142–3145. https://doi.org/10.1016/j.bmcl.2010.03.091
Carbó R, Calabuig B (1989) Molsimil-88: molecular similarity calculations using a CNDO-like approximation. Comput Phys Commun 55(1):117–126
Cheminformatics M (2011) Calculation of molecular properties and bioactivity score. https://www.molinspiration.com/docu/miscreen/druglikeness.html
Chen HP, Zhao YT, Zhao TC (2015) Histone deacetylases and mechanisms of regulation of gene expression. Crit Rev Oncog 20(1–2):35–47. https://doi.org/10.1615/critrevoncog.2015012997
Cheng Y, He C, Wang M, Ma X, Mo F, Yang S, Han J, Wei X (2019) Targeting epigenetic regulators for cancer therapy: mechanisms and advances in clinical trials. Signal Transduct Target Ther 4(1):62. https://doi.org/10.1038/s41392-019-0095-0
Davidchack RL, Handel R, Tretyakov MV (2009) Langevin thermostat for rigid body dynamics. J Chem Phys. https://doi.org/10.1063/1.3149788
DeLano WL (2002) Pymol: an open-source molecular graphics tool. CCP4 Newsl Protein Crystallogr 40(1):82–92
Demircioğlu Z, Kaştaş ÇA, Büyükgüngör O (2015) Theoretical analysis (NBO, NPA, Mulliken population method) and molecular orbital studies (hardness, chemical potential, electrophilicity and Fukui function analysis) of (E)-2-((4-hydroxy-2-methylphenylimino)methyl)-3-methoxyphenol. J Mol Struct 1091:183–195. https://doi.org/10.1016/j.molstruc.2015.02.076
Dsilva P, Pai P, Shetty MG, Babitha KS (2023) The role of histone deacetylases in embryonic development. Mol Reprod Dev 90(1):14–26. https://doi.org/10.1002/mrd.23659
Fatima N, Baqri SSR, Bhattacharya A, Koney NK-K, Husain K, Abbas A, Ansari RA (2021) Role of flavonoids as epigenetic modulators in cancer prevention and therapy. Front Genet. https://doi.org/10.3389/fgene.2021.758733
Gahtori R, Tripathi AH, Kumari A, Negi N, Paliwal A, Tripathi P, Joshi P, Rai RC, Upadhyay SK (2023) Anticancer plant-derivatives: deciphering their oncopreventive and therapeutic potential in molecular terms. Future J Pharm Sci 9(1):14. https://doi.org/10.1186/s43094-023-00465-5
Ganesan A (2020) Targeting the zinc-dependent histone deacetylases (HDACs) for drug discovery. In: Mai A (ed) Chemical epigenetics. Springer International Publishing, Cham, pp 1–27. https://doi.org/10.1007/7355_2019_68
Ghosh DC, Bhattacharyya S (2004) Molecular orbital and density functional study of the formation, charge transfer, bonding and the conformational isomerism of the boron trifluoride (BF3) and ammonia (NH3) donor-acceptor complex. Int J Mol Sci 5(8):239–264
Govindarajan M, Karabacak M, Suvitha A, Periandy S (2012) FT-IR, FT-Raman, ab initio, HF and DFT studies, NBO, HOMO–LUMO and electronic structure calculations on 4-chloro-3-nitrotoluene. Spectrochim Acta Part A Mol Biomol Spectrosc 89:137–148. https://doi.org/10.1016/j.saa.2011.12.067
Gujral P, Mahajan V, Lissaman AC, Ponnampalam AP (2020) Histone acetylation and the role of histone deacetylases in normal cyclic endometrium. Reprod Biol Endocrinol 18(1):84. https://doi.org/10.1186/s12958-020-00637-5
Hai R, Yang D, Zheng F, Wang W, Han X, Bode AM, Luo X (2022) The emerging roles of HDACs and their therapeutic implications in cancer. Eur J Pharmacol 931:175216. https://doi.org/10.1016/j.ejphar.2022.175216
Hamze A (2020) How do we improve histone deacetylase inhibitor drug discovery? Expert Opin Drug Discov 15(5):527–529. https://doi.org/10.1080/17460441.2020.1736032
Hsu KC, Chen YF, Lin SR, Yang JM (2011) iGEMDOCK: a graphical environment of enhancing GEMDOCK using pharmacological interactions and post-screening analysis. BMC Bioinform 12(Suppl 1):S33. https://doi.org/10.1186/1471-2105-12-s1-s33
Huertas J, Schöler HR, Cojocaru V (2021) Histone tails cooperate to control the breathing of genomic nucleosomes. PLoS Comput Biol 17(6):e1009013. https://doi.org/10.1371/journal.pcbi.1009013
Jorgensen WL, Chandrasekhar J, Madura JD, Impey RW, Klein ML (1983) Comparison of simple potential functions for simulating liquid water. J Chem Phys 79(2):926–935. https://doi.org/10.1063/1.445869
Karagiannis D, Rampias T (2021) HDAC inhibitors: dissecting mechanisms of action to counter tumor heterogeneity. Cancers. https://doi.org/10.3390/cancers13143575
Karton A, Spackman PR (2021) Evaluation of density functional theory for a large and diverse set of organic and inorganic equilibrium structures. J Comput Chem 42(22):1590–1601. https://doi.org/10.1002/jcc.26698
Kee HJ, Kim I, Jeong MH (2022) Zinc-dependent histone deacetylases: potential therapeutic targets for arterial hypertension. Biochem Pharmacol 202:115111. https://doi.org/10.1016/j.bcp.2022.115111
Kılıç-Cıkla I, Güveli Ş, Yavuz M, Bal-Demirci T, Ülküseven B (2016) 5-Methyl-2-hydroxy-acetophenone-thiosemicarbazone and its nickel(II) complex: crystallographic, spectroscopic (IR, NMR and UV) and DFT studies. Polyhedron 105:104–114. https://doi.org/10.1016/j.poly.2015.12.021
Kumar P, Bhardwaj VK, Purohit R (2023) Dispersion-corrected DFT calculations and umbrella sampling simulations to investigate stability of Chrysin-cyclodextrin inclusion complexes. Carbohydr Polym 319:121162. https://doi.org/10.1016/j.carbpol.2023.121162
Kumar P, Bhardwaj VK, Purohit R (2024) Highly robust quantum mechanics and umbrella sampling studies on inclusion complexes of curcumin and β-cyclodextrin. Carbohydr Polym 323:121432. https://doi.org/10.1016/j.carbpol.2023.121432
Lee C, Yang W, Parr RG (1988) Development of the Colle–Salvetti correlation-energy formula into a functional of the electron density. Phys Rev B 37(2):785–789. https://doi.org/10.1103/PhysRevB.37.785
Li G, Tian Y, Zhu WG (2020) The roles of histone deacetylases and their inhibitors in cancer therapy. Front Cell Dev Biol 8:576946. https://doi.org/10.3389/fcell.2020.576946
Liu YM, Liou JP (2023) An updated patent review of histone deacetylase (HDAC) inhibitors in cancer (2020–present). Expert Opin Ther Pat 33(5):349–369. https://doi.org/10.1080/13543776.2023.2219393
Losson H, Schnekenburger M, Dicato M, Diederich M (2016) Natural compound histone deacetylase inhibitors (HDACi): synergy with inflammatory signaling pathway modulators and clinical applications in cancer. Molecules (basel, Switzerland). https://doi.org/10.3390/molecules21111608
Lu Y, Chan Y-T, Tan H-Y, Li S, Wang N, Feng Y (2020) Epigenetic regulation in human cancer: the potential role of epi-drug in cancer therapy. Mol Cancer 19(1):79. https://doi.org/10.1186/s12943-020-01197-3
Luo Y, Li H (2020) Structure-based inhibitor discovery of class I histone deacetylases (HDACs). Int J Mol Sci. https://doi.org/10.3390/ijms21228828
Luque FJ, López JM, Orozco M (2000) Perspective on “Electrostatic interactions of a solute with a continuum. A direct utilization of ab initio molecular potentials for the prevision of solvent effects.” Theor Chem Acc 103(3):343–345. https://doi.org/10.1007/s002149900013
Mangal M, Sagar P, Singh H, Raghava GP, Agarwal SM (2013) NPACT: naturally occurring plant-based anti-cancer compound-activity-target database. Nucleic Acids Res 41(Database Issue):D1124-1129. https://doi.org/10.1093/nar/gks1047
Milazzo G, Mercatelli D, Di Muzio G, Triboli L, De Rosa P, Perini G, Giorgi FM (2020) Histone deacetylases (HDACs): evolution, specificity, role in transcriptional complexes, and pharmacological actionability. Genes 11(5):556
Morris GM, Huey R, Lindstrom W, Sanner MF, Belew RK, Goodsell DS, Olson AJ (2009) AutoDock4 and AutoDockTools4: automated docking with selective receptor flexibility. J Comput Chem 30(16):2785–2791. https://doi.org/10.1002/jcc.21256
Naeem A, Hu P, Yang M, Zhang J, Liu Y, Zhu W, Zheng Q (2022) Natural products as anticancer agents: current status and future perspectives. Molecules 27(23):8367
Neese F (2012) The ORCA program system. Wiley Interdiscip Rev Comput Mol Sci 2(1):73–78
Neese F (2022) Software update: the ORCA program system—Version 5.0. 12(5):e1606.https://doi.org/10.1002/wcms.1606
Parisi S, Piscitelli S, Passaro F, Russo T (2020) HMGA proteins in stemness and differentiation of embryonic and adult stem cells. Int J Mol Sci. https://doi.org/10.3390/ijms21010362
Pettersen EF, Goddard TD, Huang CC, Couch GS, Greenblatt DM, Meng EC, Ferrin TE (2004) UCSF Chimera—a visualization system for exploratory research and analysis. J Comput Chem 25(13):1605–1612. https://doi.org/10.1002/jcc.20084
Porter NJ, Christianson DW (2019) Structure, mechanism, and inhibition of the zinc-dependent histone deacetylases. Curr Opin Struct Biol 59:9–18. https://doi.org/10.1016/j.sbi.2019.01.004
Pramanik SD, Kumar Halder A, Mukherjee U, Kumar D, Dey YN (2022) Potential of histone deacetylase inhibitors in the control and regulation of prostate, breast and ovarian cancer. Front Chem. https://doi.org/10.3389/fchem.2022.948217
Roy R, Ria T, RoyMahaPatra D, Sk UH (2023) Single inhibitors versus dual inhibitors: role of HDAC in cancer. ACS Omega 8(19):16532–16544. https://doi.org/10.1021/acsomega.3c00222
Schäfer A, Horn H, Ahlrichs R (1992) Fully optimized contracted Gaussian basis sets for atoms Li to Kr. J Chem Phys 97(4):2571–2577. https://doi.org/10.1063/1.463096
Scrocco E, Tomasi J (1978) Electronic molecular structure, reactivity and intermolecular forces: an euristic interpretation by means of electrostatic molecular potentials. In: Löwdin P-O (ed) Advances in quantum chemistry, vol 11. Academic Press, pp 115–193. https://doi.org/10.1016/S0065-3276(08)60236-1
Shah A, Jain M (2022) Chapter 9—limitations and future challenges of computer-aided drug design methods. In: Rudrapal M, Egbuna C (eds) Computer aided drug design (CADD): from ligand-based methods to structure-based approaches. Elsevier, pp 283–297. https://doi.org/10.1016/B978-0-323-90608-1.00006-X
Shanmugam G, Rakshit S, Sarkar K (2022) HDAC inhibitors: targets for tumor therapy, immune modulation and lung diseases. Transl Oncol 16:101312. https://doi.org/10.1016/j.tranon.2021.101312
Singh R, Purohit R (2023) Computational analysis of protein-ligand interaction by targeting a cell cycle restrainer. Comput Methods Programs Biomed 231:107367. https://doi.org/10.1016/j.cmpb.2023.107367
Singh AK, Bishayee A, Pandey AK (2018) Targeting histone deacetylases with natural and synthetic agents: an emerging anticancer strategy. Nutrients. https://doi.org/10.3390/nu10060731
Sülsen VP, Athanassopoulos CM, Padrón JM, Tamura RE (2022) Editorial: natural compounds as scaffolds for the discovery of new anti-cancer drugs: focus on terpenoids and flavonoids. Front Pharmacol. https://doi.org/10.3389/fphar.2022.984849
Ullah A, Munir S, Badshah SL, Khan N, Ghani L, Poulson BG, Emwas AH, Jaremko M (2020) Important flavonoids and their role as a therapeutic agent. Molecules (basel, Switzerland). https://doi.org/10.3390/molecules25225243
Vijayakumar T, Hubert Joe I, Reghunadhan Nair CP, Jayakumar VS (2008) Efficient π electrons delocalization in prospective push–pull non-linear optical chromophore 4-[N, N-dimethylamino]-4′-nitro stilbene (DANS): a vibrational spectroscopic study. Chem Phys 343(1):83–99. https://doi.org/10.1016/j.chemphys.2007.10.033
von Szentpály L, Kaya S, Karakuş N (2020) Why and when is electrophilicity minimized? New theorems and guiding rules†. J Phys Chem A 124(51):10897–10908. https://doi.org/10.1021/acs.jpca.0c08196
Weigend F, Ahlrichs R (2005) Balanced basis sets of split valence, triple zeta valence and quadruple zeta valence quality for H to Rn: design and assessment of accuracy. Phys Chem Chem Phys 7(18):3297–3305. https://doi.org/10.1039/b508541a
Yadav R, Mishra P, Yadav D (2019) Histone deacetylase inhibitors: a prospect in drug discovery. Turk J Pharm Sci 16(1):101–114. https://doi.org/10.4274/tjps.75047
Zhong L, Li Y, Xiong L, Wang W, Wu M, Yuan T, Yang W, Tian C, Miao Z, Wang T, Yang S (2021) Small molecules in targeted cancer therapy: advances, challenges, and future perspectives. Signal Transduct Target Ther 6(1):201. https://doi.org/10.1038/s41392-021-00572-w
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
AS and AC conceptualization, methodology, resources and writing of manuscript. MJ MD simulation studies. SP DFT studies. VP, GP and AP analysis of data and final editing. All the authors have read and approved final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declared no conflict of interest.
Research involving human participants and/or animals
Not applicable.
Informed consent
Not applicable.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Shah, A., Choudhary, A., Jain, M. et al. Discovery of novel anticancer flavonoids as potential HDAC2 inhibitors: virtual screening approach based on molecular docking, DFT and molecular dynamics simulations studies. 3 Biotech 14, 83 (2024). https://doi.org/10.1007/s13205-023-03912-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13205-023-03912-5