Abstract
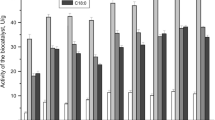
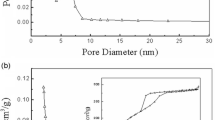
This study deals with lipase immobilization on micro- and mesoporous silica-based materials. The effects of the type of support (silica MCM-41, zeolite HZSM-5 (SAR 25), zeolite HZSM-5 (SAR 280), and the silica-aluminas Siral 10, Siral 20, and Siral 40) were investigated on the immobilization of lipase B from Candida antarctica (CALB) and lipase from Rhizomucor miehei (RML). The supports that allowed the highest immobilization efficiencies for the CALB were Siral 40 (91.4%), HZSM-5 (SAR 280) (90.6%), and MCM-41 (89.4%). Siral 20 allowed the highest immobilization efficiency for RML (97.6%), followed by HZSM-5 (SAR 25) (77.1%) and HZSM-5 (SAR 280) (62.7%). The effect of protein concentration on lipase immobilization was investigated, and the results adjusted well on the Langmuir isotherm model (R2 > 0.9). The maximum protein adsorption capacity of the support determined by the Langmuir model was equal to 10.64 and 20.97 mgprotein gsupport−1 for CALB and RML, respectively. The effects of pH (pH 7.0 and pH 11.0) and phosphate buffer solution concentration (5 and 100 mmol L−1) were also investigated on lipase immobilization. The immobilization efficiency for both lipases was similar for the different pH values. The use of 100 mmol L−1 phosphate buffer decreased the lipase immobilization efficiency. The biocatalysts (CALB-Siral 40 and RML-Siral 20) were tested in the ethyl oleate synthesis. The conversion of 61.7% was obtained at 60 °C in the reaction catalyzed by CALB-Siral 40. Both heterogeneous biocatalysts showed increased thermal stability compared with their free form. Finally, the reuse of the biocatalysts was studied. CALB-Siral 40 and RML-Siral 20 maintained about 30% of the initial conversion after 3 batches of ethyl oleate synthesis. Silica-aluminas (Siral 20 and 40) proved to be a support that allowed a high efficiency of immobilization of lipases and activity for esterification reaction.











Similar content being viewed by others
Data availability
Not applicable.
References
Aarthy M, Saravanan P, Gowthaman MK et al (2014) Enzymatic transesterification for production of biodiesel using yeast lipases: an overview. Chem Eng Res Des 92:1591–1601. https://doi.org/10.1016/j.cherd.2014.04.008
Aguieiras ECG, Souza SL, Langone MA (2013) Estudo do comportamento da lipase comercial lipozyme RM IM em reações de esterificação para obtenção de biodiesel. Quim Nova 36:646–650
Aguieiras ECG, Ribeiro DS, Couteiro PP et al (2016) Investigation of the reuse of immobilized lipases in biodiesel synthesis: influence of different solvents in lipase activity. Appl Biochem Biotechnol 179:485–496. https://doi.org/10.1007/s12010-016-2008-9
Al-Duri B, Yong YP (2000) Lipase immobilisation: an equilibrium study of lipases immobilised on hydrophobic and hydrophilic/hydrophobic supports. Biochem Eng J 4:207–215. https://doi.org/10.1016/S1369-703X(99)00050-9
Alves MD, Aracri FM, Cren ÉC, Mendes AA (2017) Isotherm, kinetic, mechanism and thermodynamic studies of adsorption of a microbial lipase on a mesoporous and hydrophobic resin. Chem Eng J 311:1–12. https://doi.org/10.1016/j.cej.2016.11.069
Amini Z, Ilham Z, Ong HC et al (2017) State of the art and prospective of lipase-catalyzed transesterification reaction for biodiesel production. Energy Convers Manag 141:339–353. https://doi.org/10.1016/j.enconman.2016.09.049
Ansorge-Schumacher MB, Thum O (2013) Immobilised lipases in the cosmetics industry. Chem Soc Rev 42:6475–6490. https://doi.org/10.1039/c3cs35484a
Antonopoulou I, Varriale S, Topakas E et al (2016) Enzymatic synthesis of bioactive compounds with high potential for cosmeceutical application. Appl Microbiol Biotechnol 100:6519–6543. https://doi.org/10.1007/s00253-016-7647-9
Baerlocher C, McCusker LB (2007) New advances in zeolite structure analysis. Stud Surf Sci Catal 170:657–665. https://doi.org/10.1016/S0167-2991(07)80905-0
Bajaj A, Lohan P, Jha PN, Mehrotra R (2010) Biodiesel production through lipase catalyzed transesterification: an overview. J Mol Catal B Enzym 62:9–14
Barsé LQ, Graebin NG, Cipolatti EP et al (2019) Production and optimization of isopropyl palmitate via biocatalytic route using home-made enzymatic catalysts. J Chem Technol Biotechnol 94:389–397. https://doi.org/10.1002/jctb.5782
Barth A (2007) Infrared spectroscopy of proteins. Biochim Biophys Acta Bioenerg 1767:1073–1101. https://doi.org/10.1016/j.bbabio.2007.06.004
Bassi JJ, Todero LM, Lage FAP et al (2016) Interfacial activation of lipases on hydrophobic support and application in the synthesis of a lubricant ester. Int J Biol Macromol 92:900–909. https://doi.org/10.1016/j.ijbiomac.2016.07.097
Bezbradica D, Mijin D, Šiler-Marinković S, Knežević Z (2007) The effect of substrate polarity on the lipase-catalyzed synthesis of aroma esters in solvent-free systems. J Mol Catal B Enzym 45:97–101. https://doi.org/10.1016/j.molcatb.2006.12.003
Bornscheuer UT (2002) Microbial carboxyl esterases: classi¢cation, properties and application in biocatalysis. FEMS Microbiol Rev 26:73–81
Boudrant J, Woodley JM, Fernandez-Lafuente R (2020) Parameters necessary to define an immobilized enzyme preparation. Process Biochem 90:66–80. https://doi.org/10.1016/j.procbio.2019.11.026
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254. https://doi.org/10.1016/0003-2697(76)90527-3
Brady D, Jordaan J (2009) Advances in enzyme immobilisation. Biotechnol Lett 31:1639–1650
Brautaset T, Ellingsen TE (2011) Comprehensive biotechnology. Elsevier
Brito MJP, Veloso CM, Bonomo RCF et al (2017) Activated carbons preparation from yellow mombin fruit stones for lipase immobilization. Fuel Process Technol 156:421–428. https://doi.org/10.1016/j.fuproc.2016.10.003
Campisano ISP, de Queiros EE, de Oliveira VC et al (2021) Solvent-free lipase-catalyzed synthesis of linear and thermally stable polyesters obtained from diacids and diols. Brazilian J Chem Eng 38:549–562. https://doi.org/10.1007/s43153-021-00137-y
Chandra P, Enespa Singh R, Arora PK (2020) Microbial lipases and their industrial applications: a comprehensive review. BioMed Central 19:1–42
Chen NY (1976) Hydrophobic properties of zeolites. J Phys Chem 80:60–64. https://doi.org/10.1021/j100542a013
Christopher LP, Kumar H, Zambare VP (2014) Enzymatic biodiesel: challenges and opportunities. Appl Energy 119:497–520
Cipolatti EP, Silva MJA, Klein M et al (2014) Current status and trends in enzymatic nanoimmobilization. J Mol Catal B Enzym 99:56–67. https://doi.org/10.1016/j.molcatb.2013.10.019
Cipolatti EP, Moreno-pérez S, Tereza L et al (2015) Synthesis and modification of polyurethane for immobilization of Thermomyces lanuginosus ( TLL ) lipase for ethanolysis of fish oil in solvent free system. J Mol Catal B Enzym 122:163–169. https://doi.org/10.1016/j.molcatb.2015.09.006
Cipolatti EP, Valério A, Henriques RO et al (2020) Production of new nanobiocatalysts via immobilization of lipase B from C. antarctica on polyurethane nanosupports for application on food and pharmaceutical industries. Int J Biol Macromol 165:2957–2963. https://doi.org/10.1016/j.ijbiomac.2020.10.179
Ćorović M, Milivojević A, Simović M et al (2020) Enzymatically derived oil-based L-ascorbyl esters: synthesis, antioxidant properties and controlled release from cosmetic formulations. Sustain Chem Pharm. https://doi.org/10.1016/j.scp.2020.100231
Costantini A, Califano V (2021) Lipase immobilization in mesoporous silica nanoparticles for biofuel production. Catalysts. https://doi.org/10.3390/catal11050629
Cunha AG, Besteti MD, Manoel EA et al (2014) Preparation of core-shell polymer supports to immobilize lipase B from Candida antarctica: effect of the support nature on catalytic properties. J Mol Catal B Enzym 100:59–67. https://doi.org/10.1016/j.molcatb.2013.11.020
da Dutra L, Pinto MCC, Cipolatti EP et al (2022) How the biodiesel from immobilized enzymes production is going on: an advanced bibliometric evaluation of global research. Renew Sustain Energy Rev 153:111765. https://doi.org/10.1016/j.rser.2021.111765
Date AA, Nagarsenker MS (2008) Parenteral microemulsions: an overview. Int J Pharm 355:19–30. https://doi.org/10.1016/J.IJPHARM.2008.01.004
Dumitriu E, Secundo F, Patarin J, Fechete I (2003) Preparation and properties of lipase immobilized on MCM-36 support. J Mol Catal B Enzym 22:119–133. https://doi.org/10.1016/S1381-1177(03)00015-8
Everton SS, Sousa I, da Silva DL et al (2022) The role of Brazil in the advancement of enzymatic biodiesel production. Brazil J Chem Eng. https://doi.org/10.1007/s43153-022-00229-3
Fasim A, More VS, More SS (2021) Large-scale production of enzymes for biotechnology uses. Curr Opin Biotechnol 69:68–76. https://doi.org/10.1016/j.copbio.2020.12.002
Fernandez-Lafuente R, Armisén P, Sabuquillo P et al (1998) Immobilization of lipases by selective adsorption on hydrophobic supports. Chem Phys Lipids 93:185–197. https://doi.org/10.1016/S0009-3084(98)00042-5
Foresti ML, Ferreira ML (2005) Solvent-free ethyl oleate synthesis mediated by lipase from Candida antarctica B adsorbed on polypropylene powder. Catal Today 107–108:23–30. https://doi.org/10.1016/J.CATTOD.2005.07.053
Foresti ML, Valle G, Bonetto R et al (2010) FTIR, SEM and fractal dimension characterization of lipase B from Candida antarctica immobilized onto titania at selected conditions. Appl Surf Sci 256:1624–1635. https://doi.org/10.1016/J.APSUSC.2009.09.083
Garcia-galan C, Berenguer-Murcia Á, Fernandez-Lafuente R, Rodrigues RC (2011) Potential of different enzyme immobilization strategies to improve enzyme performance. Adv Synth Catal 353:2885–2904. https://doi.org/10.1002/adsc.201100534
Gog A, Roman M, Toşa M et al (2012) Biodiesel production using enzymatic transesterification - current state and perspectives. Renew Energy 39:10–16
Gustafsson H, Johansson EM, Barrabino A et al (2012) Immobilization of lipase from Mucor miehei and Rhizopus oryzae into mesoporous silica–the effect of varied particle size and morphology. Colloids Surf B Biointerfaces 100:22–30. https://doi.org/10.1016/j.colsurfb.2012.04.042
Hanefeld U, Gardossi L, Magner E (2009) Understanding enzyme immobilisation. Chem Soc Rev 38:453–468. https://doi.org/10.1039/b711564b
Hensen EJM, Poduval DG, Ligthart DAJM et al (2010) Quantification of strong brønsted acid sites in aluminosilicates. J Phys Chem C 114:8363–8374. https://doi.org/10.1021/jp9106348
Hua YW, Fang M, Tong D, shen, et al (2013) Immobilization of Candida rugosa lipase on hexagonal mesoporous silicas and selective esterification in nonaqueous medium. Biochem Eng J 70:97–105. https://doi.org/10.1016/j.bej.2012.10.005
Jesionowski T, Zdarta J, Krajewska B (2014) Enzyme immobilization by adsorption: a review. Adsorption 20:801–821. https://doi.org/10.1007/s10450-014-9623-y
José C, Bonetto RD, Gambaro LA et al (2011) Investigation of the causes of deactivation–degradation of the commercial biocatalyst Novozym® 435 in ethanol and ethanol–aqueous media. J Mol Catal B Enzym 71:95–107. https://doi.org/10.1016/J.MOLCATB.2011.04.004
Kalantari M, Yu M, Yang Y et al (2017) Tailoring mesoporous-silica nanoparticles for robust immobilization of lipase and biocatalysis. Nano Res 10:605–617. https://doi.org/10.1007/s12274-016-1320-6
Kim H, Yoon SH, Choi HN et al (2006) Amperometric glucose biosensor based on sol-gel-derived zirconia / nafion composite film as encapsulation matrix. Bull Korean Chem Soc 27:65–70
Kong J, Yu S (2007) Fourier transform infrared spectroscopic analysis of protein secondary structures. Acta Biochim Biophys Sin (Shanghai) 39:549–559. https://doi.org/10.1111/j.1745-7270.2007.00320.x
Krajewska B (2004) Application of chitin- and chitosan-based materials for enzyme immobilizations: a review. Enzyme Microb Technol 35:126–139. https://doi.org/10.1016/j.enzmictec.2003.12.013
Kumar A, Verma V, Dubey VK et al (2023) Industrial applications of fungal lipases: a review. Front Microbiol. https://doi.org/10.3389/fmicb.2023.1142536
Li N, Bai R (2005) Copper adsorption on chitosan–cellulose hydrogel beads: behaviors and mechanisms. Sep Purif Technol 42:237–247. https://doi.org/10.1016/J.SEPPUR.2004.08.002
Ma HZ, Yu XW, Song C et al (2016) Immobilization of Candida Antarctica lipase B on epoxy modified silica by sol-gel process. J Mol Catal B Enzym 127:76–81. https://doi.org/10.1016/J.MOLCATB.2016.02.014
Macario A, Giordano G, Setti L et al (2007) Study of lipase immobilization on zeolitic support and transesterification reaction in a solvent free-system. Biocatal Biotransformation 25:328–335. https://doi.org/10.1080/10242420701444256
Macario A, Katovic A, Giordano G, et al (2005) Immobilization of Lipase on microporous and mesoporous materials: Studies of the support surfaces. In: Studies in Surface Science and Catalysis. Elsevier Inc., pp 381–394
Manoel EA, Dos Santos JCS, Freire DMG et al (2015a) Immobilization of lipases on hydrophobic supports involves the open form of the enzyme. Enzyme Microb Technol 71:53–57. https://doi.org/10.1016/j.enzmictec.2015.02.001
Manoel EA, José CS, Freire DMG et al (2015b) Immobilization of lipases on hydrophobic supports involves the open form of the enzyme. Enzyme Microb Technol 71:53–57. https://doi.org/10.1016/j.enzmictec.2015.02.001
Manoel EA, Pinto M, dos Santos JCS et al (2016) Design of a core–shell support to improve lipase features by immobilization. RSC Adv 6:62814–62824. https://doi.org/10.1039/C6RA13350A
Mateo C, Palomo JM, Fernandez-Lorente G et al (2007) Improvement of enzyme activity, stability and selectivity via immobilization techniques. Enzyme Microb Technol 40:1451–1463. https://doi.org/10.1016/j.enzmictec.2007.01.018
Mitchell S, Pérez-Ramírez J (2011) Mesoporous zeolites as enzyme carriers: synthesis, characterization, and application in biocatalysis. Catal Today 168:28–37. https://doi.org/10.1016/j.cattod.2010.10.058
Mokhtar NF, Raja Noor Zaliha RNZR, Muhd Noor ND et al (2020) The immobilization of lipases on porous support by adsorption and hydrophobic interaction method. Catalysts 10:1–17. https://doi.org/10.3390/catal10070744
Nakamoto H, Takahashi H (1982) Hydrophobic natures of zeolite ZSM-5. Zeolites 2:67–68. https://doi.org/10.1016/S0144-2449(82)80002-X
Pedro KCNR, Ferreira IEP, Henriques CA, Langone MAP (2019) Enzymatic fatty acid ethyl esters synthesis using acid soybean oil and liquid lipase formulation. Chem Eng Commun 207:43–55. https://doi.org/10.1080/00986445.2019.1572001
Pinto MCC, Everton SS, Cirilo LCM et al (2020) Effect of hydrophobicity degree of polymer particles on lipase immobilization and on biocatalyst performance. Biocatal Biotransformation 0:1–11. https://doi.org/10.1080/10242422.2020.1739026
Rashmi BS, Gayathri D (2014) Partial purification, characterization of Lactobacillus sp. G5 lipase and their probiotic potential. Int Food Res J 21:1737–1743
Reis P, Holmberg K, Watzke H et al (2009) Lipases at interfaces: a review. Adv Colloid Interface Sci 147–148:237–250
Rodrigues RC, Ortiz C, Berenguer-Murcia Á et al (2013) Modifying enzyme activity and selectivity by immobilization. Chem Soc Rev 42:6290–6307. https://doi.org/10.1039/c2cs35231a
Rodrigues RC, Virgen-Ortíz JJ, dos Santos JCS et al (2019) Immobilization of lipases on hydrophobic supports: immobilization mechanism, advantages, problems, and solutions. Biotechnol Adv 37:746–770. https://doi.org/10.1016/J.BIOTECHADV.2019.04.003
Sá AGA, de Meneses AC, de Araújo PHH, de Oliveira D (2017) A review on enzymatic synthesis of aromatic esters used as flavor ingredients for food, cosmetics and pharmaceuticals industries. Trends Food Sci Technol 69:95–105. https://doi.org/10.1016/j.tifs.2017.09.004
Secundo F, Roda G, Vittorini M et al (2011) Effect of chemical composition of SBA-15 on the adsorption and catalytic activity of α-chymotrypsin. J Mater Chem 21:15619–15628. https://doi.org/10.1039/c1jm11475a
Serralha FN, Lopes JM, Lemos F et al (1998) Zeolites as supports for an enzymatic alcoholysis reaction. J Mol Catal B Enzym 4:303–311. https://doi.org/10.1016/S1381-1177(98)00069-1
Sheldon RA, van Pelt S (2013) Enzyme immobilisation in biocatalysis: why, what and how. Chem Soc Rev 42:6223–35. https://doi.org/10.1039/c3cs60075k
Stergiou PY, Foukis A, Filippou M et al (2013) Advances in lipase-catalyzed esterification reactions. Biotechnol Adv 31:1846–1859. https://doi.org/10.1016/j.biotechadv.2013.08.006
Talbert JN, Goddard JM (2012) Enzymes on material surfaces. Colloids Surfaces B Biointerfaces 93:8–19. https://doi.org/10.1016/j.colsurfb.2012.01.003
Tan T, Lu J, Nie K et al (2010) Biodiesel production with immobilized lipase : a review. Biotechnol Adv. https://doi.org/10.1016/j.biotechadv.2010.05.012
Thangaraj B, Solomon PR (2019) Immobilization of lipases - a Review. Part II: carrier materials. Chem Bioeng Rev 6:167–194. https://doi.org/10.1002/cben.201900017
Tsutsumi K, Kawai T, Yanagihara T (1994) Adsorption characteristics of hydrophobic zeolites. Stud Surf Sci Catal 83:217–224. https://doi.org/10.1016/S0167-2991(08)63260-7
Uppenberg J, Hansen MT, Patkar S, Jones TA (1994) The sequence, crystal structure determination and refinement of two crystal forms of lipase B from Candida antarctica. Structure 2:293–308. https://doi.org/10.1016/S0969-2126(00)00031-9
Van De Weert M, Haris PI, Hennink WE, Crommelin DJA (2001) Fourier transform infrared spectrometric analysis of protein conformation: effect of sampling method and stress factors. Anal Biochem 297:160–169. https://doi.org/10.1006/ABIO.2001.5337
Vescovi V, Kopp W, Guisán JM et al (2016) Improved catalytic properties of Candida antarctica lipase B multi-attached on tailor-made hydrophobic silica containing octyl and multifunctional amino- glutaraldehyde spacer arms. Process Biochem 51:2055–2066. https://doi.org/10.1016/J.PROCBIO.2016.09.016
Wang C, feng, Zhou G wei, Li YJ, et al (2012) Biocatalytic esterification of caprylic acid with caprylic alcohol by immobilized lipase on amino-functionalized mesoporous silica. Colloids Surfaces A Physicochem Eng Asp 406:75–83. https://doi.org/10.1016/j.colsurfa.2012.04.053
Wenchao W, Fashe L, Ying L (2020) Effect of biodiesel ester structure optimization on low temperature performance and oxidation stability. J Mater Res Technol 9:2727–2736. https://doi.org/10.1016/J.JMRT.2020.01.005
Yu Z, Jameel H, Chang H et al (2013b) Quantification of bound and free enzymes during enzymatic hydrolysis and their reactivities on cellulose and lignocellulose. Bioresour Technol 147:369–377. https://doi.org/10.1016/j.biortech.2013.08.010
Zhou Z, Hartmann M (2013) Progress in enzyme immobilization in ordered mesoporous materials and related applications. Chem Soc Rev 42:3894–3912. https://doi.org/10.1039/c3cs60059a
Acknowledgements
The authors thank UFRJ, UERJ, and IFRJ for supporting, and thank Fundação de Amparo à Pesquisa do Estado do Rio de Janeiro- FAPERJ (E-26/211.889/2021, E-26/200.144/2023, E-26/201.366/2021) for funding the research.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there is no conflict of interest.
Ethical standards
Tests on humans or animals were not carried out in the research. All authors are aware of and approve the submission of the work.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Pedro, K.C.N.R., da Silva, J.V.V., Cipolatti, E.P. et al. Adsorption of lipases on porous silica-based materials for esterification in a solvent-free system. 3 Biotech 13, 380 (2023). https://doi.org/10.1007/s13205-023-03801-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13205-023-03801-x