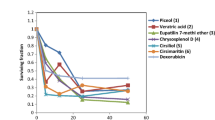
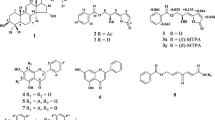
Abstract
Diarylheptanoids are a major class of plant secondary metabolites characterized by 1, 7-diphenyl heptanes in a seven-member carbon frame. In the present study, diarylheptanoids (garuganins 1, 3, 4 and 5) isolated from Garuga pinnata stem bark were evaluated for cytotoxic activity against MCF-7 and HCT15 cancer cell lines. Among the tested compounds, garuganin 5 and 3 exhibited the highest cytotoxic activity against HCT15 and MCF-7 with IC50 2.9 ± 00.8 μg/mL, 3.3 ± 0.1 μg/mL and 3.2 ± 0.1 μg/mL, and 3.5 ± 0.3 μg/mL, respectively. The molecular docking of garuganin 1, 3, 4 and 5 exhibited significant affinity toward the tested EGFR 4Hjo protein. The free energy and inhibitory constant of the compounds ranged from − 7.47 to − 8.49 kcal/mol and 3.34 micromolar to 944.20 nM nanomolar, respectively. Based on the results of cytotoxic activity, garuganin 5 and 3 were further evaluated for time- and concentration-dependent intracellular accumulation studies. The time-dependent intracellular concentration of garuganin 3 and 5 after 5 h of incubation increased about 5.5- and 4.5-fold, 204.16 ± 0.02 and 145.4 ± 0.36 nmol/L mg, respectively. The concentration-dependent intracellular concentration of garuganin 3 and 5 at 200 µg/mL increased of about > 12- and ninefold, 186.22 ± 0.05 and 98.73 ± 0.02 nmol/L mg, respectively. The intracellular concentrations of garuganin 3 and 5, in the presence of verapamil, cyclosporine and MK 571, was found to be significant in the basal direction compared to the apical directions. The results indicate that, garuganin 3 and 5 exhibited significant cytotoxic activity against MCF-7 and HCT15 cancer cell lines and also exhibited high binding affinity toward EGFR protein compared to garuganin 1 and 4.




Similar content being viewed by others
Data availability statement
Data are available on request only due to ethical, legal, or commercial reasons.
References
Alberti A, Riethmuller E, Beni S (2018) Characterization of diarylheptanoids: an emerging class of bioactive natural products. J Pharm Biomed Anal 147:13–34. https://doi.org/10.1016/j.jpba.2017.08.051
Andl CD, Mizushima T, Oyama K, Bowser M, Nakagawa H, Rustgi AK (2004) EGFR-induced cell migration is mediated predominantly by the JAK-STAT pathway in primary esophageal keratinocytes. Am J Physiol Liver Physiol 287:G1227–G1237. https://doi.org/10.1152/ajpgi.00253.2004. (20)
Arora A, Scholar EM (2005) Role of tyrosine kinase inhibitors in cancer therapy. J Pharmacol Exp Ther 315:971–979. https://doi.org/10.1124/jpet.105.084145
Avni GD, Ghulam NQ, Ramesh KG, Mahmoud ET, Singh J, Ajit KS (2008) Medcinal plants and cancer chemoprevention. Curr Drug Metab 9:581–591. https://doi.org/10.2174/138920008785821657
Dassault Systèmes BIOVIA, R (2016) Discovery studio modeling environment. Dassault Systèmes, San Diego
Fei M, Wei N, Xiang X, Hui W, Jing W, Min J, Jian L (2016) Chemical structure-related drug-like criteria of global approved drugs. Molecules 21:75. https://doi.org/10.3390/molecules21010075
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR et al (2009) Gaussian 09, Revision B01. Gaussian, Inc., Wallingford
Högnason T, Chatterjee S, Vartanian T, Ratan RR, Ernewein KM, Habib AA (2001) Epidermal growth factor receptor induced apoptosis: potentiation by inhibition of Ras signalling. FEBS Lett 491:9–15. https://doi.org/10.1016/s0014-5793(01)02166-4
Ishida J, Kozuka M, Tokuda H, Nishino H, Nagumo S, Lee KH, Nagai M (2002) Chemopreventive potential of cyclic diarylheptanoids. Bioorg Med Chem 10:3361–3365. https://doi.org/10.1016/s0968-0896(02)00164-5
Keseru GM, Nogradi M (1993) Prediction of antibacterial activity of some diarylheptanoids isolated from Garuga species by molecular mechanics and molecular orbital calculations. J Mol Struct Theochem 286:259–265. https://doi.org/10.1016/0166-1280(93)87168-D
Khatun MT, Siddiqi MMA, Al-Mansur MA, Sohrab MH, Rahman AM, Hasan CM, Chowdhury AS (2013) New diarylheptanoid from Garuga pinnata roxb Dhaka University. J Sci 11:165–167. https://doi.org/10.3329/dujps.v11i2.14575
Kunnumakkara AB, Bordoloi D, Padmavathi G, Monisha J, Roy NK, Prasad S, Aggarwal BB (2017) Curcumin, the golden nutraceutical: multitargeting for multiple chronic diseases. Br J Pharmacol 174:1325–1348. https://doi.org/10.1111/bph.13621
Lee O, Kim J, Choi YW, Lee M, Park G, Oh C (2013) Efficacy of oregonin investigated by non-invasive evaluation in a B16 mouse melanoma model. Exp Dermatol 22:842–849. https://doi.org/10.1111/exd.12277
Ma Q, Kim EY, Han O (2010) Bioactive dietary polyphenols decrease heme iron absorption by decreasing basolateral iron release in human intestinal Caco-2 cells. J Nutr 140:1117–1121
Palabindela R, Guda R, Ramesh G, Bodapati R, Nukala SK, Myadaraveni P, Ravi G, Kasula M (2023) Curcumin based pyrazole-thiazole hybrids as antiproliferative agents: synthesis, pharmacokinetic, photophysical properties, and docking studies. J Mol Struct 1275:134633
Plumb JA, Milroy R, Kaye SB (1989) Effects of the pH dependence of 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-tetrazolium bromide-formazan absorption on chemosensitivity determined by a novel tetrazoliumbased assay. Cancer Res 49:4435–4440
Ravez S, Six P, Chavatte P, Garofalo A, Farce A, Lemoine A, Goossens L, Depreux P (2012) Synthesis and structure-activity relationships of (Aryloxy) quinazoline ureas as novel, potent, and selective vascular endothelial growth factor receptor-2 inhibitors. J Med Chem 55:1189–1204. https://doi.org/10.1021/jm2013453
Su Ki L, Sharan M, Norhafiza MA, Noor HN (2020) A review of the structure—activity relationship of natural and synthetic antimetastatic compounds. Biomolecules 10:138. https://doi.org/10.3390/biom10010138
Tabernero J (2007) The role of VEGF and EGFR inhibition: implications for combining anti-VEGF and anti-EGFR agents. Mol Cancer Res 5:203–220. https://doi.org/10.1158/1541-7786.MCR-06-0404
Thupurani MK, Reddy PN, Singara Charya MA, Thirupathaiah A, Shiva D (2012) In vitro determination of antioxidant activities of Garuga pinnata Roxb. Int J Med Arom Plants 2:566–572
Thupurani MK, Reddy PN, Singara Charya MA (2013a) In vitro and in vivo antidiabetic potentialities of Garuga pinnata Roxb stem bark. Int J Pharm Ther 4:255–260
Thupurani MK, Reddy PN, Pardhasaradhi M, Ventka Raman B, Singara Charya MA (2013b) Studies on anticancer and antibacterial potentialities of Garuga pinnata roxb. Int J Pharm Sci Rev Res 21:163–167
Thupurani MK, Reddy PN, SingaraCharya MA, Geethanjali M (2013c) In vitro and in vivo determination of anti-inflammatory activities of Garuga pinnata Roxb. Int J Pharm Sci Rev Res 22:310–314
Uto T, Tung NH, Appiah OR, Aning A, Morinaga O, Edoh D, Nyarko AK, Shoyama Y (2015) Antiproliferative and pro-apoptotic activity of diarylheptanoids isolated from the bark of Alnus japonica in humanleukemia cell lines. Am J Chin Med 43:757–767. https://doi.org/10.1142/S0192415X15500470
Vaidyanathan JB, Walle T (2003) Cellular uptake and efflux of the tea flavonoid (-)-epicatechin-3-gallate in the human intestinal cell line Caco-2. J Pharmacol Exp Ther 307:745–752. https://doi.org/10.1124/jpet.103.054296
Vidakovic V, Novakovic M, Popovic Z, Jankovic M, Matic R, Tesevic V, Bojovic S (2017) Significance of diarylheptanoids for chemotaxonomical distinguishing between Alnus glutinosa and Alnus incana. Holzforschung 72:9–16. https://doi.org/10.1515/hf-2017-0074
Wieduwilt MJ, Moasser MM (2008) The epidermal growth factor receptor family: biology driving targeted therapeutics. Cell Mol Life Sci 65:1566–1584. https://doi.org/10.1007/s00018-008-7440-8
Xi Y, Gao H, Callaghan MU, Fribley AM, Garshott DM, Xu ZX, Zeng Q, Li YL (2015) Induction of BCL2-Interacting killer BIK, is mediated for anti-cancer activity of curcumin in human head and neck squamous cell carcinomacells. J Cancer 6:327–332. https://doi.org/10.7150/jca.11185
Yang L, Wencui SA, Mohammad SA, Adnan AK, Yurngdong J, Youngjoo K, Motiur Rahman AFM (2020) Synthesis, biological evaluation and molecular docking study of cyclic diarylheptanoids as potential anticancer therapeutics. Anti-Cancer Agents Med Chem 20:464–475. https://doi.org/10.2174/1871520619666191125130237
Yun CH, Boggon TJ, Li Y, Woo MS, Greulich H, Meyerson M, Eck MJ (2007) Structures of lung cancer-derived EGFR mutants and inhibitor complexes: mechanism of activation and insights into differential inhibitor sensitivity. Cancer Cell 11:217–227. https://doi.org/10.1016/j.ccr.2006.12.017
Acknowledgements
The authors are grateful to the Chancellor, Chaitnya Deemed to be University, for his cooperation and encouragement and also thankful to the Department of Biotechnology, NIT Warangal, for providing facilities for this research work.
Funding
The research leading to these results received funding from the Science and Engineering Research Board (SERB), New Delhi, India, under the project of TARE (Teachers Associateship for Research Excellence). Grant. FILE NO.TAR/2018/000561.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors disclose no conflict of financial or nonfinancial interest.
Ethical statements
The authors do not have any potential conflict of interest. This study does not involve any human beings or animals.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Konakanchi, S., Vadluri, R., Anumula, K.S. et al. Antiproliferative, molecular docking, and bioavailability studies of diarylheptanoids isolated from stem bark of Garuga pinnata Rox B.. 3 Biotech 13, 208 (2023). https://doi.org/10.1007/s13205-023-03581-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13205-023-03581-4