Abstract
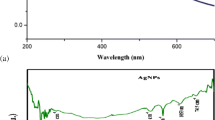
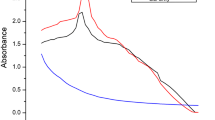
In the current study, extracellular biosynthesis of silver nanoparticles (AgNPs) was carried out using aqueous extracts of green Calligonum comosum stem, besides Fusarium sp. Synthesized AgNPs were characterized using ultraviolet (UV)–Vis spectrophotometer, transmission electron microscopy (TEM) and zeta potential. Moreover, biosynthesized AgNPs were estimated for the scavenging ability on DPPH radical as well as tested for their antibacterial activity using well diffusion method against Gram-positive bacteria Staphylococcus aureus. On the other hand, DNA content from untreated and AgNPs treated bacterial cells was evaluated by (UV)–Vis spectrophotometer and agarose gel electrophoresis. Results revealed the formation of AgNPs, which was first detected by color change of the reaction mixture. The characteristic surface plasmon resonance absorption was detected at 450 and 410 nm for the plant and myco-synthesized AgNPs. Furthermore, TEM micrograph and zeta sizer showed formation of spherical particles with an average size of about 105.8 and 228.4 nm for plant and myco-synthesized AgNPs, respectively. Plant-synthesized AgNPs exhibited higher scavenging of DPPH radicals than that of the myco-synthesized one. For bactericidal action, plant-synthesized AgNPs showed higher inhibition zone compared with myco-synthesized one, which was negatively correlated with the nanoparticle size. Furthermore, low DNA concentration was detected for AgNPs treated bacteria, which might be a consequence of inactivation for DNA replication. Further experimental work is required to find out if there is any correlation between nanoparticles size and efficacy against bacteria.






Similar content being viewed by others
References
Azizi S, Rosfarizan M, Mahnaz MS (2017) Green microwave-assisted combustion synthesis of zinc oxide nanoparticles with Citrullus colocynthis (L.) Schrad: characterization and biomedical applications. Molecules 2:301
Banerjee P, Satapathy M, Mukhopahayay A, Das P (2014) Leaf extract mediated green synthesis of silver nanoparticles from widely available Indian plants: synthesis, characterization, antimicrobial property and toxicity analysis. Bioresour Bioprocess 1:3
Blois MS (1958) Antioxidant determinations by the use of a stable free radical. Nature 181:1199–1200
Danilcauk M, Lund A, Saldo J, Yamada H, Michalik J (2006) Conduction electron spin resonance of small silver particles. Spectrochim Acta A 63(75):189–191
Deepak V, Kalimuthu K, Sureshbabu RKP, Gurunathan S (2011) Deepak et al book chapter. In: Rai MK, Duran N (eds) Metal nanoparticles in microbiology. Springer, New York, pp 17–35
dos Santos CA, Seckler MM, Ingle AP, Gupta I, Galdiero S, Galdiero M, Gade A, Rai M (2014) Silver nanoparticles: therapeutical uses, toxicity and safety issues. J Pharm Sci 103:1931–1944
Duran N, Marcato PD, Alves OL, De Souza GIH, Esposito E (2005) Mechanistic aspects of biosynthesis of silver nanoparticles by several Fusarium oxysporum strains. J Nano Biotechnol 3:8–14
Franci G, Falanga A, Galdiero S, Palomba L, Rai M, Morelli G, Galdiero M (2015) Silver nanoparticles as potential antibacterial agents. Molecules 20:8856–8874. https://doi.org/10.3390/molecules20058856
Ghazanfar SA (ed) (1994) Handbook of Arabian medicinal plants. CRC Press, Boca Raton, p 173
Heydari R, Rashidipour M (2015) Green synthesis of silver nanoparticles using extract of Oak Fruit Hull (Jaft): synthesis and in vitro cytotoxic effect on MCF-7 cells. Int J Breast Cancer 2015:846743
Honary S, Barabadi H, Gharaei-Fathabad E, Naghibi F (2013) Green synthesis of silver nanoparticles induced by the fungus Penicillium citrinum. Trop J Pharm Res 12(1):7–11
Ingle A, Gade A, Pierrat S, Sonnichsen C, Rai MK (2008) Mycosynthesis of silver nanoparticles using the fungus Fusarium acuminatum and its activity against some human pathogenic bacteria. Curr Nanosci 4:141–144
Ishida K, Cipriano TF, Rocha GM, Weissmüller G, Gomes F, Miranda K, Rozental S (2014) Silver nanoparticle production by the fungus Fusarium oxysporum: nanoparticle characterisation and analysis of antifungal activity against pathogenic yeasts. Mem Inst Oswaldo Cruz 109(2):220–228
Jemal K, Sandeep BV, Pola S (2017) Synthesis, characterization, and evaluation of the antibacterial activity of Allophylus serratus leaf and leaf derived callus extracts mediated silver nanoparticles. J Nanomater. https://doi.org/10.1155/2017/4213275
Jha AK, Prasad K, Kulkami AR (2009) Plant system: nature’s nanofactory. Colloids Surf B 73:219–223
Kamil M, Jayaraj AF, Ahmad F, Gunasekhar C, Samuel S, Habiballah M, Chan K (2000) Pharmacognostic and phytochemical standardisation of Calligonum comosum. J Pharm Pharm 52(Suppl):262
Kim J, Kuk E, Yu K, Kim JH, Park SJ, Lee HJ, Kim SH, Park YK, Park YH, Hwang C-Y, Kim YK, Lee YS, Jeong DH, Cho MH (2007) Antimicrobial effects of silver nanoparticles. Nanomedicine 3:95–101
Macdonald IDG, Smith WE (1996) Orientation of cytochrome C adsorbed on a citrate-reduced silver colloid surface. Langmuir 12:706–713
Mohammed AE (2015) Green synthesis and antimicrobial activity of Eucalyptus camaldulensis mediated silver nanoparticles. Asian Pac J Trop Biomed 5:930–934
Mukherjee P, Ahmad A, Mandal D, Senapati S, Sainkar RS, Khan IM, Parishcha R, Ajaykumar VP, Alam M, Kumar R, Sastry M (2001) Fungus-mediated synthesis of silver nanoparticles and their immobilization in the mycelia matrix. A novel biological approach to nanoparticle synthesis. Nano Lett 1(10):515–519
Naveen HKS, Gaurav K, Karthik L, Rao BKV (2010) Extracellular biosynthesis of silver nanoparticles using the filamentous fungus Penicillium sp. Arch App Sci Res 2(6):161–167
Ouda SM (2014) Some nanoparticles effects on Proteus sp. and Klebsiella sp. isolated from water. Am J Infect Dis Microbiol 2:4–10
Paulkumar K, Gnanajobitha G, Vanaja M, Rajeshkumar S, Malarkodi C, Pandian K, Annadurai G (2014) Piper nigrum leaf and stem assisted green synthesis of silver nanoparticles and evaluation of its antibacterial activity against agricultural plant pathogens. Sci World J. https://doi.org/10.1155/2014/829894
Pelgrift RY, Friedman AJ (2013) Nanotechnology as a therapeutic tool to combat microbial resistance. Adv Drug Deliv Rev 65:1803–1815
Prabhu S, Poulose EK (2012) Silver nanoparticles: mechanism of antimicrobial action, synthesis, medical applications, and toxicity effects. Int Nano Lett 2:1–10
Rai RV, Bai JA (2011) Science against microbial pathogens: communicating current research and technological advances. In: Méndez-Vilas A (ed) Nanoparticles and their potential application as antimicrobials. University of Mysore, Manasagangotri, Mysore
Ratnasri PV, Hemalatha KPJ (2014) Biological synthesis of silver nanoparticles from Aspergillus fumigatus. Am J Drug Deliv 2:6
Raudabaugh DB, Tzolov MB, Calabrese JP, Overton BE (2013) Synthesis of silver nanoparticles by a Bryophilous rhizoctonia species. Nanomater Nanotechnol 3:2
Sahayaraj K, Rajesh S (2011) Science against microbial pathogens: communicating current research and technological advances. In: Méndez-Vilas A (ed) Bionanoparticles: synthesis and antimicrobial applications. Formatex Research Center, Badajoz
Sastry M, Ahmad A, Khan MI, Kumar R (2003) Biosynthesis of metal nanoparticles using fungi and actinomycete. Curr Sci 85(2):162–170
Singh P, Raja RB (2011) Biological synthesis and characterization of silver nanoparticles using the fungus Trichoderma harzianum. Asian J Exp Biol Sci 2(4):600
Singh Kh, Panghal M, Kadyan S, Yadav JP (2014) Evaluation of antimicrobial activity of synthesized silver nanoparticles using Phyllanthus amarus and Tinospora cordifolia medicinal plants. J Nanomed Nanotechnol 5:6
Tamayo LA, Zapata PA, Vejar ND, Azocar MI, Gulppi MA, Zhou X, Thompson GE, Rabagliati FM, Paez MA (2014) Release of silver and copper nanoparticles from polyethylene nanocomposites and their penetration into Listeria monocytogenes. Mater Sci Eng C 40:24–31
Thorley AJ, Tetley TD (2013) New perspectives in nano-medicine. Pharmacol Ther 140:176–185
Umoren SA, Obot IB, Gasem ZM (2014) Green synthesis and characterization of silver nanoparticles using red apple (Malus domestica) fruit extract at room temperature. J Mater Environ Sci 5(3):907–914
Vahabi KH, Mansoori GA, Sedighe K (2011) Biosynthesis of silver nanoparticles by fungus Trichoderma reesei. Int Sci J 1(1):65–79. https://doi.org/10.5640/insc.010165
Vahdati AR, Sadeghi B (2013) A study on the assessment of DNA strand-breaking activity by silver and silica nanoparticles. J Nanostruck Chem 3:7. http://www.jnanochem.com/content/3/1/7
Wu D, Fan W, Kishen A, Gutmann JL, Fan B (2014) Evaluation of the antibacterial efficacy of silver nanoparticles against Enterococcus faecalis biofilm. J Endod 40:285–290
Yang H, Yang SH, Kong J, Dong A, Yu Sh (2015) Obtaining information about protein secondary structures in aqueous solution using Fourier transform IR spectroscopy. Nat Protoc 10(3):382–396
Aknowledgements
Authors are grateful to staff members in the Biology Section, Faculty of Science, PNU for their continuous support. Thankfullness is also extended to King Faisal specialist hospital and research center for providing the necessary facilities during the experimental period of this research work.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The author has no competing interest.
Rights and permissions
About this article
Cite this article
Mohammed, A.E., Bin Baz, F.F. & Albrahim, J.S. Calligonum comosum and Fusarium sp. extracts as bio-mediator in silver nanoparticles formation: characterization, antioxidant and antibacterial capability. 3 Biotech 8, 72 (2018). https://doi.org/10.1007/s13205-017-1046-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13205-017-1046-5