Abstract
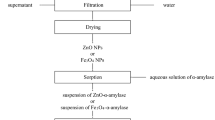
The role of enzyme engineering in biotechnology, biological and pharmaceutical process cannot be over emphasized. This study compared the adsorption of digestives enzymes; amylase, protease and lipase on to Zn-ferrite (ZnFe2O4). The metal ferrite was synthesized via a sol–gel technique and characterized with scanning electron microscopy (SEM), X-ray diffraction (XRD), Electron paramagnetic resonance (EPR) and Fourier transform infrared spectroscopy (FTIR). The adsorption was studied in a batch process and the data were subjected to kinetics and isotherm models. Characterization shows that the particle has a nanoporous structure, with pore sizes of about 5.4 nm and good magnetic properties. The FTIR data showed the presence of M–O bond, which is a characteristic of metal ferrites. The adsorption of the amylase, lipase and protease on ZnFe2O4 follow first-order kinetic model with rate constants increasing with concentration. The maximum adsorption capacities as revealed by the generalized adsorption isotherms are 7.20, 42.90 and 22.24 mg g−1 for amylase, lipase and protease, respectively, with cooperative binding. The Dubinin–Radushkevich model gave the maximum adsorption energies, E of 3.74 kJ mol−1 for amylase, 2.01 kJ mol−1 for lipase and 1.51 kJ mol−1 for the protease adsorption, showing that the process is physisorption dominated. The isotherms fit the adsorption data in the order of Freundlinch > Generalized > Guggenheim–Anderson–de Boer > Tempkin isotherm > Dubinin–Radushkevich. Thermodynamic study revealed a spontaneous adsorption process with increased entropy. ZnFe2O4, therefore, is a very good adsorbent for the purification of enzymes and can be used as a supporter for enzymatic process that required immobilization of the enzymes.









Similar content being viewed by others
References
Adeogun AI, Babu RB (2015) One-step synthesized calcium phosphate-based material for the removal of alizarin S dye from aqueous solutions: isothermal, kinetics, and thermodynamics studies. Appl Nanosci 5:1–13
Adeogun AI, Balakrishnan RB (2016) Electrocoagulation removal of anthraquinone dye Alizarin Red S from aqueous solution using aluminum electrodes: kinetics, isothermal and thermodynamics studies. J Electrochem Sci Eng 6(2):199–213
Assarsson A, Nasir I, Lundqvist M, Cabaleiro-Lago C (2016) Kinetic and thermodynamic study of the interactions between human carbonic anhydrase variants and polystyrene nanoparticles of different size. RSC Adv 6(42):35868–35874
Blanco RM, Alvaro G, Guisán J (1991) Enzyme reaction engineering: design of peptide synthesis by stabilized trypsin. Enzyme Microb Technol 13(7):573–583
Bornhorst JA, Falke JJ (2000) Purification of proteins using polyhistidine affinity tags. Methods Enzymol 326:245–254
Burns RG (1976) The uptake of cobalt into ferromanganese nodules, soils, and synthetic manganese (IV) oxides. Geochim Cosmochim Acta 40(1):95–102
Crini G, Peindy HN (2006) Adsorption of C.I. Basic Blue 9 on cyclodextrin-based material containing carboxylic groups. Dyes Pigment 70(3):204–211
Do DD (1998) Adsorption analysis: equilibria and kinetics: (With CD Containing Computer Matlab Programs), vol 2. World Scientific, Singapore
Gooding JJ, Hall EAH (1996) Membrane properties of acrylate bulk polymers for biosensor applications. Biosens Bioelectron 11(10):1031–1040
Huang XL, Catignani GL, Swaisgood HE (1997) Comparison of the properties of trypsin immobilized on 2 Celite™ derivatives. J Biotechnol 53(1):21–27
Kargi F, Ozmıhcı S (2004) Biosorption performance of powdered activated sludge for removal of different dyestuffs. Enzyme Microb Technol 35(2):267–271
Kumar P, Sharma SM (2015) An overview of purification methods for proteins. IJAR 1(12):450–459
Lagergren S (1996) Zur theorie der sogenannten adsorption gelöster stoffe. Bil. K. Svenska Venteskapsakad, Handl. 24 (1898) as cited by Wasay et al. Water Res 30:1143–1148
Lemus MR (2011) Models of sorption isotherms for food: uses and limitations. Vitae 18(3):325–334
MacCormack TJ, Clark RJ, Dang MK, Ma G, Kelly JA, Veinot JG, Goss GG (2012) Inhibition of enzyme activity by nanomaterials: potential mechanisms and implications for nanotoxicity testing. Nanotoxicology 6(5):514–525
McNamara K, Tofail SA (2017) Nanoparticles in biomedical applications. Adv Phys X 2(1):54–88
Meissner J, Prause A, Bharti B, Findenegg GH (2015) Characterization of protein adsorption onto silica nanoparticles: influence of pH and ionic strength. Colloid Polym Sci 293(11):3381–3391
Nune SK, Gunda P, Thallapally PK, Lin YY, Laird Forrest M, Berkland CJ (2009) Nanoparticles for biomedical imaging. Expert Opin Drug Deliv 6(11):1175–1194
Özacar M, Şengil İA (2005) A kinetic study of metal complex dye sorption onto pine sawdust. Process Biochem 40(2):565–572
Robinson PK (2015) Enzymes: principles and biotechnological applications. Essays Biochem 59:1–41
Safarik I, Safarikova M (2004) Magnetic techniques for the isolation and purification of proteins and peptides. BioMagn Res Technol 2(1):7
Sarmiento F, Peralta R, Blamey JM (2015) Cold and hot extremozymes: industrial relevance and current trends. Front Bioeng Biotechnol 3:148
Slocik JM, Naik RR (2010) Probing peptide–nanomaterial interactions. Chem Soc Rev 39(9):3454–3463
Tütem E, Apak R, Ünal ÇF (1998) Adsorptive removal of chlorophenols from water by bituminous shale. Water Res 32(8):2315–2324
Umut E (2013) Surface modification of nanoparticles used in biomedical applications. Mod Surf Eng Treat 1:85–208
Weber WJ, Morris JC (1963) Kinetics of adsorption on carbon from solution. J Sanit Eng Div 89(2):31–60
Xu J, Sun J, Wang Y, Sheng J, Wang F, Sun M (2014) Application of iron magnetic nanoparticles in protein immobilization. Molecules 19(8):11465–11486
Yildiz A, Gür A (2007) Adsorption of phenol and chlorophenols on pure and modified sepiolite. J Serb Chem Soc 72(5):467–474
Zhang J, Stanforth R (2005) Slow adsorption reaction between arsenic species and goethite (α-FeOOH): diffusion or heterogeneous surface reaction control. Langmuir 21:2895–2901
Zheng W, Li XM, Wang F, Yang Q, Deng P, Zeng GM (2008) Adsorption removal of cadmium and copper from aqueous solution by areca—A food waste. J Hazard Mater 157(2–3):490–495
Acknowledgements
This work benefitted immensely from the financial support in the form of grants from CSIR, for 12 months TWAS-CSIR Postdoctoral Fellowship, FR Number: 3240275035, awarded to Abideen Idowu Adeogun that enables part of this work to be carried out at the CECRI Pollution Control Division Laboratory. We are also he is thankful to the authority of the Federal University of Agriculture, Abeokuta, Nigeria for Granting the study leave to honor the fellowship.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Rights and permissions
About this article
Cite this article
Adeogun, A.I., Kareeem, S.O., Adebayo, O.S. et al. Comparative adsorption of amylase, protease and lipase on ZnFe2O4: kinetics, isothermal and thermodynamics studies. 3 Biotech 7, 198 (2017). https://doi.org/10.1007/s13205-017-0859-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13205-017-0859-6