Abstract
Removal of long-chain hydrocarbons and n-alkanes from oil-contaminated environments are mere important to reduce the ecological damages, while bio-augmentation is a very promising technology that requires highly efficient microbes. In present study, the efficiency of pure isolates, i.e., Geobacillus thermoparaffinivorans IR2, Geobacillus stearothermophillus IR4 and Bacillus licheniformis MN6 and mixed consortium on degradation of long-chain n-alkanes C32 and C40 was investigated by batch cultivation test. Biodegradation efficiencies were found high for C32 by mixed consortium (90%) than pure strains, while the pure strains were better in degradation of C40 than mixed consortium (87%). In contrast, the maximum alkane hydroxylase activities (161 µmol mg−1 protein) were recorded in mixed consortium system that had supplied with C40 as sole carbon source. Also, the alcohol dehydrogenase (71 µmol mg−1 protein) and lipase activity (57 µmol mg−1 protein) were found high. Along with the enzyme activities, the hydrophobicity natures of the bacterial strains were found to determine the degradation efficiency of the hydrocarbons. Thus, the study suggested that the hydrophobicity of the bacteria is a critical parameter to understand the biodegradation of n-alkanes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Oil contamination and pollution is a global issue that poses threat to the ecology and environment (Hasanuzzaman et al. 2007; Zhang et al. 2012a; Kumar et al. 2016). The crude oil and refinery compounds released into the environment consists of major polluting compounds of different carbon chain lengths straight n-alkanes, branched (isoalkanes), or cyclic (naphthenes) arrangement up to 60 carbons and aromatic hydrocarbons (Abrajano and Yan 2014). United States National Research Council classified these organic contaminants into high and low molecular weight hydrocarbons, oxygenated, halogenated aliphatics, aromatics, nitro aromatics hydrocarbons and alkanes (NRC 2000; Abrajano and Yan 2014). These hydrocarbons and alkanes have been considered as priority pollutants (NRC 2000; Ekperusi and Aigbodion 2015), which are biohazards and biomagnified in the environment (Kramer and Van der Heijden 1990; Mohamed et al. 2006). While, there are special group of microorganisms that can biodegrade and biotransform these hydrocarbons and alkanes to assimilate as biomass in the ecosystem (Chikere et al. 2012; Rajasekar et al. 2012; Roy et al. 2015; Kumar et al. 2016). However, few alkanes are recalcitrant in the environment (e.g., long chain and high molecular weight hydrocarbons) due to their non-polar and chemically inert nature (Labinger and Bercaw 2002). Extensive research studies and reviews reported the biodegradation of the short chain hydrocarbons, while very limited studies reported the alkane and long-chain hydrocarbon degradations, especially under thermophilic conditions (Sakai et al. 1994; Ko and Lebeault 1999; Hasanuzzaman et al. 2007).
Thermophilic bacterial strains play a vital role in the degradation of aliphatic hydrocarbons than mesophilic groups thus significance to use it for bio-augmented cleaning of oil-contaminated environment (Wang et al. 2011). Many of the thermophilic bacterial genera such as Thermomicrobium sp., Bacillus sp., Geobacillus sp., and Thermus sp. were identified and characterized from different hydrocarbon habitats (Sorkhoh et al. 1993; Nazina et al. 2001; Meintanis et al. 2006; Liu et al. 2009). Thermus sp. shows higher degradation rates of the hexadecane and benzo[e]pyrene both aliphatic and polyaromatic hydrocarbon at 70 °C that was isolated from Hot springs (Feitkenhauer et al. 2003). While, Pseudomonas aeruginosa normally metabolize the alkanes through oxidation process and it has two alkane hydroxylases (alkB1 and alkB2), cytochrome P450 and other essential electron transfer proteins that allow it to metabolize long-chain alkanes (Gunasekera et al. 2013). Similarly, G. stearothermophilus are reported to contain hydroxylase enzyme and gene-encoding alkane monooxygenase are responsible for degradation of long-chain alkanes (C12–C31) (Sakai et al. 1994; Wang et al. 2006; Liu et al. 2010; Li et al. 2010). Many microbial species were used by many researchers to degrade long-chain alkanes like B. stearothermophilus, Acinetobacter sp., B. thermoleovorans, G. kaustophilus, Dietzia sp., B. licheniformis, etc. (Sorkhoh et al. 1993; Sakai et al. 1994; Kato et al. 2001; Sood and Lal 2008; Bihari et al. 2010). However, the removal efficiency of the different isolates varied due to their action mechanisms on degradation of pollutants, substrate concentrations and their bioavailability. Temperature is a key factor to regulate the gene expression, enzyme activation, degradation of pollutant and mass transfer rates, however, the major limitations comes from bioavailability, solubility of the hydrocarbons and alkanes and the hydrophobic nature of the culture (Feitkenhauer et al. 2003).
During thermophilic (aerobic) degradation the n-alkanes oxidized into primary alcohol by the oxidation of a terminal methyl group, which is further oxidized into aldehyde (Rojo 2010). Furthermore, sub-terminal oxidation is also reported for long-chain n-alkanes during biodegradation (Wentzel et al. 2007). Number of enzymes synergistically acts upon biodegradation of n-alkanes in environment were the mixed bacterial population co-exist. Few bacterial strains have similar enzymes but one or more isometric enzyme structure, i.e., P. putida hold only one alkane hydroxylase, while A. borkumensis has two alkane hydroxylases (AHy). In few isolates, both alkane hydroxylase and alcohol dehydrogenase (ADHs) has been characterized, e.g., G. thermodenitrificans (Li et al. 2010). Alcohols generated by terminal oxidation of n-alkanes are further oxidized to aldehydes by ADHs, therefore, provide complete biotransformation. Similar to AHy, there are few microbes that can excrete four different ADHs with species specificity, i.e., towards primary and secondary alcohols (Vangnai and Arp 2001; Vangnai et al. 2002). Therefore, the degradation efficiency and intermediate products from degradation of long-chain hydrocarbons and n-alkanes rely on the type of isolate used and their enzymatic oxidation properties. There are very limited studies reported on degradation of long-chain hydrocarbon C32 and C40 by thermophiles and the significant enzyme activities. To the best of our knowledge, no studies reported the degradation pattern of C40 by thermophilic isolates and mixed consortium that exhibit high assimilation rates, therefore, the study and outcome presented in this manuscript is novel.
Afore mentioned, the present study aimed to investigate the biodegradation efficiency of pure isolates G. stearothermophilus IR4, G. thermoparaffinivorans IR2, B. licheniformis MN6 and as mixed consortium to remediate the C32 and C40, which are major pollutants from oil-contaminated environment. The functions and ability of the pure isolates and mixed consortium were compared in terms of degradation efficiency and enzyme activities by batch study.
Materials and methods
Higher n-alkanes
The n-alkanes, i.e., dotricontane (C32) (product code D223107) and tetracotane (C40) (Product code 87086) and all the chemicals used in this study were analytical grade and purchased from Sigma Aldrich, India.
Media and bacterial growth conditions
The oil and produced water samples were collected from oil reservoir located in the Cauvery river basin (Latitude: 10.7694; longitude: 79.6155), Karaikal, Tamil Nadu, India. Three thermophilic bacterial strains were isolated and identified by biochemical characterization and molecular gene sequencing approaches (16S rDNA gene analysis). The selected strains G. stearothermophilus IR4, G. Thermoparaffinivorans IR2 and B. licheniformis MN6 were characterized and identified up to genus level by their morphological and partial biochemical characterization using the following: Gram staining, motility test, Voges–Proskauer, citrate utilization, indole production, methyl red test, carbohydrate fermentation, catalase, H2S production, oxidase test, starch, gelatin and lipid hydrolysis (Holt et al. 1994). The respective sequences of the three isolates were submitted to Genebank with the accession numbers of KP338614, KP338611 and KP338616, respectively. The bacterial cultures were enriched under shake flask conditions at 120 rpm at 50 °C for 24–48 h using Luria–Bertani (LB) medium prior to experiments.
Biodegradation of n-alkanes
Biodegradation study was carried out using Bushnell Hass (BH) broth and using C32 and C40 as carbon sources. The composition of the BH broth (g L−1): MgSO4 0.2; CaCl2 0.02; KPO4 1.0; K2PO4 1.0; NH4NO3 1.0; and FeCl3 0.05. Individual strains IR2, IR4 and MN6 and consortium of bacterial cells were adjusted to OD600 ~ 1.0 and supplemented with 0.1% of C32 and C40 as a sole carbon source in BH broth. The total populations in each test conditions were ~2.1 × 106 CFU mL−1 for pure isolates and mixed consortia. The flasks were incubated at 50 °C in an orbital shaker (120 rpm) over 20 days. Abiotic controls, i.e., flask without inoculation of any isolates/mixed consortium, were also run in parallel to understand the fate of pollutants. All the experiments were done in triplicate. The aliquot samples were collected on day 10 and 20 for the analysis of protein, enzymes and intermediate products.
Protein estimation
For the determination of protein, from the IR2, IR4 and MN6 biodegradation medium were collected and harvested centrifugation at 8000 rpm at 4 °C for 10 min and then washed in potassium phosphate buffer (pH 7.0) twice. The pellet further sonicated for 5 min at 0 °C with same buffer and centrifuged at 20,000 rpm at 4 °C for 25 min. The supernatant was then stored at 2 °C and the protein content was measured according to Lowry et al. (1951) at 660 nm by UV–Vis spectrophotometer using bovine serum albumin as a standard.
Alkane hydroxylase assay
The AHy experimental methodology was adopted as previously described by Mishra and Singh (2012). In Brief, the cell-free supernatant was collected by centrifugation at 8000 rpm at 10 min (Remi, C-24BL, India) and used for AHy assay. The reaction mixture contained 0.15% CHAPS buffer (pH 7.4), 20 mM Tris–HCl and, 0.1 mM NADH, 10 µL of n-hexadecane solution (1% hexadecane in 80% DMSO), and 50 µL of crude extract in 1 mL volume. Enzyme activity was tested by absorbance at 340 nm in UV–Vis spectrophotometer (Jasco V630 model UV–Vis spectrophotometer, Japan). Alkane hydroxylase activity and the amount of enzyme are calculated using the standard slope method.
Alcohol dehydrogenase assay
The ADHs assay was carried out as described by Jauhari et al. (2014). The reaction mixture contained 4 mM NAD, 1 M Tris–HCl buffer (pH 8.8), 100 µL ethanol (99% pure) and 50 µL crude extract (i.e., cell free supernatant from culture flasks) for final make up volume of 1.0 mL. Enzyme activity was measured at 340 nm in UV–Vis spectrophotometer and concentrations were calculated from standard value.
Determination of extracellular lipase
For each day, 2 mL of culture medium was taken from the shake flasks and centrifuged at 10,000 rpm for 30 min at 4 °C (Remi, C-24 BL, India). The supernatant was filtered using a 0.22 µm membrane filter and cell-free supernatant was used for the lipase assay. Consumption of 1 mL NaOH (0.01 N) was equivalent to 10 µM of fatty acid liberated from n-alkanes. One unit of lipase activity (U) was calculated as µM of fatty acid released per min. Lipase activity was expressed in unit per mL, i.e., U mL−1 (Mishra and Singh 2012).
Bacterial adhesion to hydrocarbons (BATH)
The BATH assay was used to determine the cell surface hydrophobicity of IR2, IR4 and MN6. Cell biomass were harvested by centrifugation at 8000 g for 10 min in 4 °C, washed twice with PUM buffer (Mishra and Singh 2012), and re-suspended in PUM buffer to maintain initial absorbance (at OD400) values between 0.12 and 0.14. Around 1 mL of hexadecane and 2 mL of the adjusted cell suspension were vortexed at high speed for 2 min and incubated at 30 °C for 15 min to allow the phase separation of hydrocarbons in floating layer. The lower aqueous phase was removed carefully and turbidity of the aqueous phase was measured at OD600 using UV–Vis spectrophotometer. The percentage of cells adhere (%A) with the hydrocarbons were measured using aqueous phase concentrations as given below (Rajasekar and Ting 2010).
Gas chromatography analysis
Residual C32 and C40 from each biodegradation flasks were extracted with n-hexane (Wang et al. 2006). Finally the characterization of the n-alkanes fractions and changes in functional group were qualitatively analyzed by gas chromatography–mass spectrometry (GC–MS) with the Elite column 30.0 m × 0.25 mm, 250 µm (Perkin elmer, Clarus 680, USA). The oven temperature was set as: 60 °C for 2 min, ramp 10 °C/min to 300 °C. Helium was used as carrier gas at a constant flow rate of 1 mL min−1 and 1 μL of samples were injected for analysis. The rate of biodegradation efficiency (BE) of C32 and C40 were calculated as described earlier (Rajasekar et al. 2007).
Results
Biodegradation of n-alkanes C32 and C40
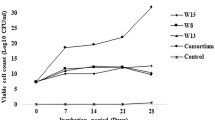
Preliminary identification of the thermophilic bacteria by biochemical test indicated that the isolates belonged to the genera Geobacillus and Bacillus. The phenotypic profiles of the thermophilic bacterial strains are shown in Table 1. Degradation of C32 and C40 by IR2, IR4 and MN6 and mixed consortium, were analyzed by GC–MS (Figs. 1, 2). The GC–MS analysis revealed that the relative abundance of each peaks were highly reduced and formed new intermediate compounds (Table 2). The isolates used in this study are highly efficient in degradation of C32 and C40 (Fig. 3). The BE of C32 was about 62% (IR2), 48% (IR4), 80% (MN6) and 85% (mixed consortium), within 10 days of incubation. Subsequent incubations improved the BE up to 90% (IR2), 82% (IR4), 87% (MN6) and 90% (mixed consortium), respectively, over 20 days (Fig. 3). Similarly, the BE of C40 was found to be 38, 42, 40 and 56% within 10 days, which further increased 82, 86, 87 and 88% for IR2, IR4, MN6 and consortium, respectively, for 20 days.
Bacterial cell protein
The bacterial protein content continued to increase with the replica of cells presence in IR2, IR4, MN6 and consortium during the incubation period. The incipient cell protein was measured between 1.08 and 1.6 mg mL−1 and then increased to a maximum of 2.42 mg mL−1 in the consortium. Individual strains did not show any major difference in the total cell protein at the end of batch incubation study which provided two different hydrocarbon sources.
Alkane hydroxylase
The maximum AHy activity in presence of C32 were measured as 122 µmol mg−1 protein (IR2), 150 µmol mg−1 protein (IR4), 143 µmol mg−1 protein (MN6) and 160 µmol mg−1 protein (mixed consortium) as shown in Fig. 4. In all test conditions, it had showed that the maximum enzyme activities were measured between 6 and 10 days and declined after 12th day towards the end. Figure 4 shows the activity of alkane hydroxylase in presence of C40 as sole carbon sources. The induction of alkane hydroxylase by the isolate were observed, IR2 (157 µmol mg−1 protein), IR4 (142 µmol mg−1 protein), MN6 (147 µmol mg−1 protein) and mixed consortium (161 µmol mg−1 protein). Each isolates shows maximum enzyme activity between 9 and 12 days of incubation. Initial high n-alkane concentrations supported the growth and higher enzyme activities of pure and mixed consortium. The AHy are membrane bound protein were reported to be mainly responsible for hydrocarbon degradation and the enzyme encoded by specific genes were reported for other pure strains in literature (Rojo 2010). However, the AHy induction revealed that initially the n-alkanes level affected the expression levels of these specific gene clusters, which were measured from the initial low concentrations of AHy at exponential growth phases of pure and mixed cultures (Canosa et al. 1999; Wentzel et al. 2007).
Alcohol dehydrogenase assay
The aerobic degradation of n-alkanes starts by the oxidation of a terminal methyl group that render a primary alcohol, via AHy enzyme in the presence of NADH rubredoxin and rubredoxin reductase, which was further dehydrogenated to aldehyde by ADHs. As shown in Fig. 5, the alcohol dehydrogenase activities are ranged maximum between 7 and 12 days during batch degradation studies, which followed the AHy induction. The maximum induction for IR2 (60 µmol mg−1 protein), IR4 (72 µmol mg−1 protein), MN6 (88 µmol mg−1 protein) and mixed consortium (89 µmol mg−1 protein) on day 11. Similarly, the ADHs in presence of C40 as sole carbon sources for IR2 (60 µmol mg−1 protein), IR4 (69 µmol mg−1 protein), MN6 (72 µmol mg−1 protein) and mixed consortium (71 µmol mg−1 protein) in 10 days of incubation (Fig. 5). In contrast with the alkane dehydrogenase, the MN6 had exhibit high ADHs activities than other pure isolates during C32 and C40 supplied as carbon source.
Lipase assay
Similar to other enzymes, the level of lipase enzyme induction was less at initial incubations and rapidly increased. Figure 6 shows the lipase activity by IR2 (34 µmol mg−1 protein), IR4 (51 µmol mg−1 protein), MN6 (38 µmol mg−1 protein) and mixed consortium (60 µmol mg−1 protein) under C32 supplied condition. While, similar trend was observed for lipase activity when the medium was supplied with C40, i.e., IR2 showed (37 µmol mg−1 protein), IR4 (41 µmol mg−1 protein), MN6 (39 µmol mg−1 protein) and mixed consortium (57 µmol mg−1 protein). Cell surface properties, changing composition of the cellular fatty acids in the presence of various hydrocarbons were reported to improve the lipophilicity and could assist the uptake, absorption and import of the hydrocarbons (Mishra and Singh 2012).
Surface hydrophobicity
Cell surface hydrophobicity (CSH) was the measure of adhesion of hydrocarbon to the cell surface. Since the initial step in hydrocarbon degradation were mediated by oxidation reactions catalyzed by cell-surface-associated oxygenase, the hydrophobic nature of the hydrocarbon and pure isolates need to be studied to explain and improve the BE (Rosenberg 1993; Fletcher 1996; Wentzel et al. 2007). From this study, the CSH was measured using the BATH assay for pure strains and mixed consortium. The hydrophobicity is about 74, 81, 78 and 86% for IR2, IR4, MN6 and mixed consortium, respectively.
Discussion
Biodegradation technology was most efficient, economic and eco-friendly approach for remediation of contaminated environments, including hydrocarbon contaminated soils. The microbes play a key role in remediation of hydrocarbon (Wang et al. 2006). In this study, we have compared the pure cultures and mixed culture for degradation of C32 and C40, while the mixed consortium showed high BE % for C32, while pure strains showed better BE % for C40. It was observed that degradation efficiency was increased with incubations period. Also, the initial growth of microbes and enzyme inductions were slightly stressed with provided sole carbon source (C32 and C40), since they were not previously been adapted to the tested carbon sources.
Moreover, the surface hydrophobicity of alkanes and bacterial cells were the crucial factor to influence the BE of pollutants in aqueous medium. In BH broth, isolates IR2, IR4 and MN6 were proved to be potentially adhering to the n-alkanes surface and utilized as carbon sources to form a new biomass (Rojo 2010). Ibrahim et al. (2013) and Gudina et al. (2012) reported that the B. licheniformis were dominant bacterial strain in oil reservoir and capable to degrade several mercaptans such as 1-decanethiol and 1-dodecanethiol at 55 °C (Chebbi et al. 2014). In this study, the MN6 was able to perform superior than other strains or mixed consortium in degradation of C40. Similarly, IR2 and IR4 were able to degrade 87–88% of C40 into different intermediate compound such as eicosane, heneicosane and docosaneetc (Table 2) (Wang et al. 2006; Zhang et al. 2012b). The degradation of C40 was found to be high and similar reports were not available in the literature yet. However, under mixed consortium, the potential bacterial synergism might have affected the degradation efficiency of C40, which required further investigation. Overall, these isolates exhibited higher BE for C32 and C40 hydrocarbon as pure or mixed consortium at thermophilic condition and promising to use for hydrocarbon remediation.
The degradation of individual hydrocarbons was also correlated with the enzyme activities and bacterial growth. Degradative enzymes such as AHy, ADHs and lipase were involved in the degradation of n-alkane C32 and C40 that were measured at different concentrations and found to be independent of the isolates and test substrates as shown in Figs. 4, 5 and 6. The membrane-bound AHy were measured in all pure isolates and also measured in other n-alkane-degrading strains (Liu et al. 2009). Alkane hydroxylase initiates the aerobic degradation of alkanes by inserting oxygen atoms at the different sites of alkane terminus (Jauhari et al. 2014; Ji et al. 2013). Such structures are exemplified by microbodies in alkane-grown bacteria (Watkinson and Morgan 1990). These alkane oxidation enzymes also reported to have diverse substrate ranges or different induction patterns (Hasanuzzaman et al. 2007; Meintanis et al. 2006). As observed in this study, the inductions of AHy were good at stationary growth phase of bacteria Fig. 4, rather than exponential growth phase.
The fatty acid alcohols generated by terminal oxidation of n-alkanes were further oxidized to aldehydes by ADHs and similarly as reported from Bacillus sp., P. butanovora and G. thermodenitrificans NG80-2 (Li et al. 2008; Vangnai and Arp 2001; Vangnai et al. 2002). Thus, the ADHs were essential for growth of microorganisms in the presence of n-alkanes. In this study ADHs play a crucial role in degradation process, since its level of induction was corresponded to the degradation of n-alkane C32 and C40 (Fig. 5). The produced aldehyde was metabolized towards corresponding carboxylic acid by the produced ADHs in a BH broth. This carboxylic acid, mainly serve as a substrate for alkane degrading microbes to accumulate fatty acids by β-oxidation pathway (May and Katapodis 1990; Lal and Khanna 1996; Mishra and Singh 2012). Thus, the roles of ADHs were crucial to determine the degradability of the n-alkanes and support the growth of degrading microbes in the BH broth. As shown in Fig. 5, the IR2, IR4 and MN6 were able to release high levels of ADHs for degradation of C32 and C40. Also, the isolates also confirmed that the production of lipase in the presence of C32 and C40 substrates is unique.
The lipases are hydrolases, which act under aqueous conditions with triacylglycerols contains carboxyl esters bonds to liberate fatty acids and glycerols. More concentration of lipase was measured from mixed consortium than pure cultures. The specific role of lipase is to induce the lipolytic reactions at lipid-water interface, where the hydrocarbons are emulsified in the broth. As reported by previous researchers (Alvarez et al. 2000; Zheng et al. 2011), the IR2, IR4 and MN6 was involved in the degradation of intermediates produced at the later phase of n-alkane mineralization in this study was evident (Table 2). This further improved the CSH. Thus, the study results suggests that the CSH was essential parameter to improve the BE, while the enzyme production is a critical factor to govern the overall mineralization of these compounds were evident.
Conclusions
The three thermophilic bacterial strains G. thermoparaffinivorans IR2, G. stearothermophilus IR4 and B. licheniformis MN6 were found to be best strains for degradation of C32 and C40 from the oil-contaminated point sources. The two major factors that determines the BE of C32 and C40 were AHy and ADHs activity along with CSH. Among the three strains tested, G. stearothermophilus IR4 found to be more robust in degradation and mineralization of C40, while their complete degradation mechanism will be further investigated, while the bacterial synergy affected the BE under mixed consortium condition’s was evident from this study. Overall, to our knowledge this is the first study that report the few robust and indigenous isolate that can degrade C40 efficiently and also for bioremediation of recalcitrant compounds from the hydrocarbon-polluted environment.
References
Abrajano TA, Yan B (2014) High molecular weight petrogenic and pyrogenic hydrocarbons in aquatic environments, vol 9. Rensselaer Polytechnic Institute, Troy, NY, USA, V O’Malley, Enterprise Ireland, Glasnevin, Republic of Ireland, Elsevier, pp 475–509
Alvarez HM, Kalscheuer R, Steinbchel A (2000) Accumulation and mobilization of storage lipids by Rhodococcus opacus PD630 and Rhodococcus ruber NCIMB 40126. Appl Microbiol Biotechnol 54:8–223
Bihari Z, Szabo Z, Szvetnik A, Balazs M, Bartos P, Tolmacsov P, Zombori Z, Kiss I (2010) Characterization of a novel long-chain n-alkane-degrading strain, Dietzia sp. E1. Z Naturforsch C 65:693–700
Canosa I, Yuste L, Rojo F (1999) Role of the alternative sigma factor sigma S in expression of the AlkS regulator of the Pseudomonas oleovorans alkane degradation pathway. J Bacteriol 181:1748–1754
Chebbi A, Mnif S, Mhiri N, Jlaiel L, Sayadi S, Chamkha M (2014) A moderately thermophilic and mercaptan-degrading Bacillus licheniformis strain CAN55 isolated from gas-washing wastewaters of the phosphate industry, Tunisia. Int Biodeterior Biodegrad 94:207–213
Chikere CB, Chikere BO, Okpokwasili GC (2012) Bioreactor-based bioremediation of hydrocarbon-polluted NigerDelta marine sediment, Nigeria. 3 Biotech 2:53–66
Ekperusi OA, Aigbodion FI (2015) Bioremediation of petroleum hydrocarbons from crude oil contaminated soil with the earthworm: hyperiodrilus africanus. 3 Biotech 5:957–965
Feitkenhauer H, Muller R, Markl H (2003) Degradation of polycyclic aromatic hydrocarbons and long chain alkanes at 60-70 °C by Thermus and Bacillus spp. Biodegradation 14:367–372
Fletcher M (1996) Bacterial attachment in aquatic environments: a diversity of surfaces and adhesion strategies. In: Fletcher M (ed) Bacterial adhesion: molecular and ecological diversity. Wiley, New York, pp 1–24
Gudina EJ, Pereira JFB, Rodrigues LR, Coutinho JAP, Teixeira JA (2012) Isolation and study of microorganisms from oil samples for application in microbial enhanced oil recovery. Int Biodeterior Biodegrad 68:56–64
Gunasekera TS, Striebich RC, Mueller SS, Strobel EM, Ruiz ON (2013) Transcriptional profiling suggests that multiple metabolic adaptations are required for effective proliferation of Pseudomonas aeruginosa in jet fuel. Environ Sci Technol 47:13449–13458
Hasanuzzaman M, Ueno A, Ito H, Ito Y, Yamamoto Y, Yumoto I, Okuyama H (2007) Degradation of long-chain n-alkanes (C36 and C40) by Pseudomonas aeruginosa strain WatG. Int Biodeterior Biodegrad 59:40–43
Holt JG, Kreig NR, Sneath PHA, Stanely JT (1994) In: Williams ST (ed) Bergey’s manual of determinative bacteriology. Williams and Wilkins, Baltimore
Ibrahim ML, Ijah UJJ, Manga SB, Bilbis LS, Umar S (2013) Production and partial characterization of biosurfactant produced by crude oil degrading bacteria. Int Biodeterior Biodegrad 81:28–34
Jauhari N, Mishra S, Kumari B, Singh SN (2014) Bacteria-mediated aerobic degradation of hexacosane in vitro conditions. Bioresour Technol 170:62–68
Ji Y, Mao G, Wang Y, Bartlam M (2013) Structural insights into diversity and n-alkane biodegradation mechanism of alkane hydroxylases. Front Microbiol 4:1–13
Kato T, Haruki M, Imanaka T, Morikawa M, Kanaya S (2001) Isolation and characterization of long-chain-alkane degrading Bacillus thermoleovorans from deep subterranean petroleum reservoirs. J Biosci Bioeng 91(1):64–70
Ko SH, Lebeault JM (1999) Effect of a mixed culture on co-oxidation during the degradation of saturated hydrocarbon mixture. J Appl Microbiol 87:72–79
Kramer PGN, Van der Heijden CA (1990) Polycyclic aromatic hydrocarbons (PAH) carcinogenicity data and risk extrapolations. In: Rose J (ed) Environmental Topics. Gordon and Breach Science Publishers, New York, pp 47–57
Kumar AR, Janardhan A, Viswanath B, Monika K, Jung JY, Narasimha G (2016) Evaluation of orange peel for biosurfactant production by Bacillus licheniformis and their ability to degrade naphthalene and crude oil. 3 Biotech 6:43
Labinger J, Bercaw J (2002) Understanding and exploiting C-H bond activation. Nature 417:507–514
Lal B, Khanna S (1996) Degradation of crude oil by Acinetobacter cocloaceticus and Alcaligenes odorans. J Appl Bacteriol 81:355–362
Li L, Liu X, Yang W, Xu F, Wang W, Feng L (2008) Crystal structure of long chain alkane monooxygenase (LadA) in complex with coenzyme FMN: unveiling the long-chain alkane hydroxylase. J Mol Biol 376:453–465
Li X, Li Y, Wei D, Li P, Wang L, Feng L (2010) Characterization of broad-range aldehyde dehydrogenase involved in alkane degradation Geobacillus thermodenitrificans NG80-2. Microbiol Res 165:706–712
Liu YC, Zhou TT, Zhang J, Xu L, Zhang ZH, Shen QR, Shen B (2009) Molecular characterization of the alkB gene in the thermophilic Geobacillus sp. strain MH-1. Res Microbiol 160:560–566
Liu YC, Li LZ, Wu Y, Tian W, Zhang LP, Xu L, Shen QR, Shen B (2010) Isolation of an alkane-degrading Alcanivorax sp. strain B5 and cloning of the alkB gene. Bioresour Technol 101:310–316
Lowry OH, Roserbrough NT, Farr AC, Randall RJ (1951) Protein measurement with Folin phenol reagent. J Biol Chem 193:265–275
May SW, Katapodis AG (1990) Hydrocarbon monooxygenases system of Pseudomonas oleovorans. Methods Enzymol 188:3–9
Meintanis C, Chalkou KI, Kormas KA, Karagouni AD (2006) Biodegradation of crude oil by thermophilic bacteria isolated from a volcano island. Biodegradation 17(2):105–111
Mishra S, Singh SN (2012) Microbial degradation of n-hexadecane in mineral salt medium as mediated by degradative enzymes. Bioresour Technol 111:148–154
Mohamed ME, Al-Dousary M, Hamzah RY, Fuchs G (2006) Isolation and characterization of indigenous thermophilic bacteria active in natural attenuation of bio-hazardous petrochemical pollutants. Int Biodeterior Biodegrad 58:213–223
Nazina TN, Tourova TP, Poltaraus AB, Novikova EV, Grigoryan AA et al (2001) Taxonomic study of aerobic thermophilic bacilli descriptions of Geobacillus subterraneus gen. nov., sp. nov. And Geobacillus uzenensis sp. nov. from petroleum reservoirs and transfer of Bacillus stearothermophilus, Bacillus thermocatenulatus, Bacillus thermoleovorans, Bacillus kaustophilus, Bacillus thermoglucosidasius and Bacillus thermodenitrificans to Geobacillus as the new combinations G. stearothermophilus, G. thermocatenulatus, G. thermoleovorans, G. kaustophilus, G. thermoglucosidasius and G. thermodenitrificans. Int J Syst Evol Micrbiol 51:433–446
NRC (2000) Natural attenuation for groundwater remediation, committee on intrinsic remediation, water science and technology board, board on radioactive waste management. National Research Council, 292
Rajasekar A, Ting YP (2010) Microbial corrosion of aluminum 2024 aeronautical alloy by hydrocarbon degrading bacteria Bacillus cereus ACE4 and Serratia marcescens ACE2. Ind Eng Chem Res 49:6054–6061
Rajasekar A, Ponmariappan S, Maruthamuthu S, Palaniswamy N (2007) Bacterial degradation and corrosion of naphtha in transporting pipeline. Curr Microbiol 55:374–381
Rajasekar A, Maruthamuthu S, Ting YP, Balasubramanian R, Rahman PKSM (2012) Bacterial degradation of petroleum hydrocarbons. In: Singh SN (ed) Microbial degradation of xenobiotics. Springer, Berlin, pp 339–369
Rojo F (2010) Enzymes for aerobic degradation of alkanes. In: Timmis KN (ed) Handbook of hydrocarbon and lipid microbiology. Springer, Berlin
Rosenberg E (1993) Exploiting microbial growth on hydrocarbons—New markets. Trends Biotechnol 11:419–424
Roy S, Chandni S, Das I, Karthik L, Kumar G, Rao KVB (2015) Aquatic model for engine oil degradation by rhamnolipid producing Nocardiopsis VITSISB3. 3 Biotech 5:153–164
Sakai Y, Maeng JH, Tani Y, Kato N (1994) Use of long-chain n-alkanes (C13–C44) by an isolate, Acinetobacter sp. M-1. Biosci Biotechnol Biochem 58:2128–2130
Sood N, Lal B (2008) Isolation and characterization of a potential paraffin-wax degrading thermophilic bacterial strain Geobacillus kaustophilus TERI NSM for application in oil wells with paraffin deposition problems. Chemosphere 70:1445–1451
Sorkhoh NA, Ibrahim AS, Ghannoum MA, Radwan SS (1993) High-temperature hydrocarbon degradation by Bacillus stearothermophilus from oil-polluted Kuwaiti desert. Appl Microbiol Biotechnol 39:123–126
Vangnai AS, Arp DJ (2001) An inducible 1-butanol dehydrogenase, a quinohaemoprotein, is involved in the oxidation of butane by Pseudomonas butanovora. Microbiology 147:745–756
Vangnai AS, Arp DJ, Sayavedra Soto LA (2002) Two distinct alcohol dehydrogenases participate in butane metabolism by Pseudomonas butanovora. J Bacteriol 184:1916–1924
Wang L, Tang Y, Wang S, Liu RL, Liu MZ, Zhang Y, Liang FL, Feng L (2006) Isolation and characterization of a novel thermophilic Bacillus strain degrading long-chain n-alkanes. Extremophiles 10:347–356
Wang XB, Chi-Qiao C, Nie Y, Tang-Qin Y, Tan Y, Wu G, Wu XL (2011) Degradation of petroleum hydrocarbons (C6–C40) and crude oil by a novel Dietzia strain. Bioresour Technol 102:7755–7761
Watkinson RJ, Morgan P (1990) Physiology of aliphatic hydrocarbon-degrading microorganisms. Biodegradation 1:79–92
Wentzel A, Ellingsen TE, Kotlar HK, Zotchev SB, Holst MT (2007) Bacterial metabolism of long-chain n-alkanes. Appl Microbiol Biotechnol 76:1209–1221
Zhang XS, Xu DJ, Zhu CY, Tserennyam L, Scherr KE (2012a) Isolation and identification of biosurfactant producing and crude oil degrading Pseudomonas aeruginosa. Chem Eng J 209:138–146
Zhang J, Zhang X, Liu J, Li R, Shen B (2012b) Isolation of a thermophilic bacterium, Geobacillus sp. SH-1, capable of degrading aliphatic hydrocarbons and naphthalene simultaneously, and identification of its naphthalene degrading pathway. Bioresour Technol 124:83–89
Zheng C, He J, Wang Y, Wang M, Huang Z (2011) Hydrocarbon degradation and bioemulsifier production by thermophilic Geobacillus pallidus strains. Bioresour Technol 102:9155–9165
Acknowledgements
A. Rajasekar thankful to the Department of Biotechnology (Government of India) for awarding Ramalingaswami Re-entry Fellowship (BT/RLF/Re-entry/17/2012), Department of Science and Technology for young scientist award (SB/YS/LS-40/2013), University Grants Commission—MRP (MRP-MAJOR-MICRO-2013-31825) and Science and Engineering Research Board, Department of Science and Technology, Government of India (EEQ/2016/000449).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made.
The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder.
To view a copy of this licence, visit https://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Elumalai, P., Parthipan, P., Karthikeyan, O.P. et al. Enzyme-mediated biodegradation of long-chain n-alkanes (C32 and C40) by thermophilic bacteria. 3 Biotech 7, 116 (2017). https://doi.org/10.1007/s13205-017-0773-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13205-017-0773-y