Abstract
The nanostructured cyclically refreshable liquid amalgam film silver-based electrode (R-AgLAFE) was applied to study of the Bi(III) electrode process in the presence of 2-thiocytosine and selected ionic surfactants. The application of voltammetrictechniques (SWV, CV, DC), as well as electrochemical impedance spectroscopy (EIS) allowed the determination of the kinetic which in turn, defined the 2-thiocytosine catalytic effect and also their correlation in the presence of surfactants. The presence of mixed 2-thiocytosine-CTAB and 2-thiocytosine-SDS adsorption layers affects the mechanism and kinetics of Bi(III) ions electro-reduction process in chlorate(VII). CTAB and SDS change the dynamics of the catalytic impact of 2-thiocytosine on Bi(III) ions electro reduction. In both cases, the Bi–(RS–Hg) complex plays a key role, as it is the 2-thiocytosine that dominates in the establishment of the adsorption equilibria of the studied mixed adsorption layers.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Knowledge of the electrochemical properties of drugs is very important for more profound understanding of their metabolic pathway, or the redox processes occurring "in vivo". The study of electrode mechanisms in the presence of medications also appears to be a useful tool to optimize and individualize the therapy of these specific drugs. This may indicate the maximum effectiveness of the drug while minimizing its toxicity in terms of the use also potential environmental damage. As biologically active substances designed to act at low concentrations, they can also have adverse effects on "non-target" organisms in the environment and, due to their tendency to bioaccumulate, are a serious threat to all levels of trophic food chains, including potentially human health.
In light of the above considerations, it seems interesting to extend the study to determine the kinetics and mechanism of the electrode process in the presence of 2-thiocytosine (TC), a drug used in chemotherapy, mainly in myeloid leukemia due to its demonstrated antileukemic activity (Shahrokhian et al. 2004) and selected surfactants (Hexadecyltrimethyl ammonium bromide (cationic) (CTAB) and Sodium-1-decanesulfonate (anionic) (SDS), typical corrosion inhibitors (Fouda et al. 2017).
These surfactants can interfere effectively with the structure of the interfacial layer due to the formation of mixed adsorption layers, affecting changes in the kinetics and mechanism of the electrode process (Saba et al. 2013; Kaliszczak and Nosal-Wiercińska 2018, 2019, 2020).
A sequence of various electrochemical techniques, such as DC polarography, cyclic voltammetry CV and square wave voltammetry SWV, as well as electrochemical impedance spectroscopy (EIS) were used for this study. This allowed us to observe changes in the dynamics of the process of TC catalytic action on the electro-reduction of Bi(III) ions due to the introduction of surfactants into the system, towards inhibition. Application of the nanostructured cyclically refreshable liquid amalgam film silver-based electrode (R-AgLAFE) under the "cap-pair" effect conditions (Nosal-Wiercińska et al. 2021a) is an excellent alternative to the mercury drop electrode due to its quality and performance parameters similar to HMDE and environmental protection.
Experimental
Materials and methods
The electrochemical experiments were performed with a μAutolab type III/ GpES version 4.9 galvanostat (EcoChemie B.V., Utrecht, The Netherlands) and a programmable M165D electrode stand (mtmanko, Krakow, Poland), in a three-electrode cell containing: a working electrode—the cyclically refreshable liquid amalgam film silver-based electrode (R-AgLAFE) refreshed before each measurement with a surface area of 17.25 mm2, the reference electrode—chlorosilver (Ag/AgCl) in the 3 mol·dm−3 KCl, auxiliary electrode—platinum wire.
Details of the R-AgLAFE electrode design, operating principles, and refreshing before each measurement are presented in the paper (Nosal-Wiercińska et al. 2021a).
The solutions were thermostated at 298°K and deaerated using nitrogen. 1 mol⋅dm−3 chlorates (VII) were used as a supporting electrolyte.
The choice was imposed by poor complex making properties of ClO4− ions, their susceptibility to water structure destruction and the fact that they adsorb only to a small extent on the mercury surface (Nosal-Wiercińska 2014).
The concentration of Bi(III) ions in the studied solution was 1⋅10−3 mol⋅dm−3. Additionally, the solutions containing 2-thiocytosine (Fluka), sodium 1-decanesulfonate (Fluka) and hexadecyltrimethyl ammonium bromide (Fluka) prepared just before the measurements were applied in the investigations. The following concentrations or ranges of concentrations of organic substances were used: TC-1⋅10−3 mol⋅dm−3, SDS from 1.5·10−5 to 9·10−5 mol·dm−3 and CTAB from 1.5·10−6 to 1.5·10−5 mol·dm−3. The investigations were carried out in the systems:
-
Bi(III)—the inhibitor (SDS or CTAB),
-
Bi(III)—the catalyst (2-thiocytosine),
-
Bi(III)—the catalyst—the inhibitor.
The procedure of measurements
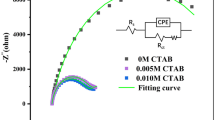
In the DC polarography, square wave voltammetry SWV and cyclic voltammetry CV, the optimal experiment operating conditions were as follows: step potential 2 mV for DC, pulse amplitude 20 mV, frequency 120 Hz and step potential 2 mV for the SWV, and scan rate 5–1000 mV s−1 and step potential 5 mV for the CV. The values of rate constants were obtained from the EIS measurements. The impedance data were collected at 36 frequencies in the range from 15 to 100,000 Hz within the faradaicpotential region with the 10 mV intervals and analyzed by the expressions valid for the Randles equivalent circuit (Sluyters-Rehbach et al. 1984). This takes into account the ohmic resistance (Re), double layer capacity (Cd), charge transfer resistance (Ra), and Wartburg element of (Zw) (Scheme 1).
To study the kinetics and mechanism of the electrode process, kinetic parameters, such as the formal potential (\({E}_{\mathrm{f}}^{0})\), reversible half-wave potential (\({E}_{1/2)}^{\upgamma }\)), transition coefficient (\(\mathrm{\alpha }),\) rate constants of the depolarizer electro-reduction process and diffusion coefficients, were determined (\({D}_{\mathrm{ox}})\). The details of the determination of the above parameters are described elsewhere (Nosal-Wiercińska et al. 2021a).
\(k_{{\text{f}}}\) values were computed from \(R_{{{\text{ct}}}}\) values as a function of dc potential (Sluyters-Rehbach et al. 1984).
where
S the electrode surface area (S = powierzchnia elektrody nasza cm2).
Results and discussion
Organic substances can inhibit, accelerate or have no effect on the electrode process (Ikeda et al. 1984; Souto et al. 1986; Dalmata 2005; Nosal-Wiercińska 2010a; Nosal-Wiercińska and Kaliszczak 2021).
Studies of the mixed adsorption layers in terms of their influence on the kinetics of depolarizer electro-reduction (Nieszporek 2011; Nieszporek and Dagci 2014; Nosal-Wiercińska et al. 2021b) have indicated changes in the dynamics of this process in the presence of a catalyst substance and a potential inhibitor. A qualitative estimation of the changing effect of TC and surfactants on the kinetics of the analyzed electrode process was confirmed using DC, SWV and CV voltammetry.
The presence of 2-thiocytosine influences the magnitude of the limiting current (Fig. 1a, b). The slope of the polarographic waves also increases, which indicates an increase in the rate of the Bi(III) electro-reduction process in the presence of the organic substance (Nosal-Wiercińska 2014). On the other hand, the addition of CTAB or SDS surfactants into the basic electrolyte solution containing TC, indicates differences in the DC curves image. The presence of CTAB does not affect the magnitude of the limiting current significantly (Fig. 1), whereas SDS (Fig. 1b) causes definite changes in the magnitude of the limiting current, especially above the concentration of 5·10−5 mol·dm−3. The indicated concentration was assigned a critical micellization concentration (Martyna et al. 2022). The determined values of (\({D}_{\mathrm{ox}})\) using the Ilkovič equation (Kaliszczak and Nosal-Wiercińska 2018) confirm the changes in solution viscosity only in the presence of SDS and above the determined CMC (Koundal et al. 2022; Refay et al. 2022). An increase in the slope of the DC wave in the presence of CTAB (at a constant TC concentration) indicates an increase in the reversibility of the electrode process, while SDS does the opposite (Fig. 1a, b) (Nosal-Wiercińska 2014). The presence of CTAB in a 1 mol·dm−3 chlorate(VII) solution containing 1·10−3 mol·dm−3 Bi(III) increases the reversibility of Bi(III) ions electro-reduction. SDS, on the other hand, causes the opposite effect, the peaks decrease, above the concentration of 5·10−5 mol·dm−3 (critical micellization concentration) they are deformed, due to the blocking of the electrode surface by formed hemimicelles (Nosal-Wiercińska et al. 2018).
a DC curves of 1·10−3 mol·dm−3 Bi(III) ions electro-reduction at the presence of 1·10−3 mol·dm−3 of 2-thiocytosine and with the influence of CTAB addition; b DC curves of 1·10−3 mol·dm−3 Bi(III) ions electro-reduction at the presence of 1·10−3 mol·dm−3 of 2-thiocytosine and with the influence of SDS addition
The same changes in the reversibility of the electro-reduction process of Bi(III) ions resulting from the image of SWV curves, for the solutions containing only surfactants can be observed (Fig. 2a–d). SWV voltamperograms confirm the effect of surfactants on the reversibility of the electrode process. The deformation of SWV peaks above a concentration of 3·10−5 mol·dm−3 for SDS was observed (Fig. 2b). The indicated concentration was attributed to the critical micellization concentration (Martyna et al. 2022) and the inhibition of the irreversible electrode reaction (\({k}_{\mathrm{s}}=\) 1.97 10−4 cm·s−1 for the electro-reduction of 1·10−3 mol dm−3 Bi(III) in 1 mol·dm−3 chlorate (VII) (Nosal-Wiercińska 2010b) to the blocking of the electrode surface by the formed hemimicelles (Kaliszczak and Nosal-Wiercińska 2018) (Fig. 2a, b). However, the presence of the studied surfactants on the SVW peaks of the electro-reduction of Bi(III) ions in 1 mol·dm−3 chlorate (VII) at a constant concentration of the catalyst substance (1·10−3 mol dm−3) indicates a further increase in the reversibility of the electro-reduction process in the presence of CTAB and a decrease in the reversibility in the presence of SDS (Nosal-Wiercińska 2014). An increase in the CTAB concentration causes an increase in the SWV peak current, with a simultaneous slight destabilization of the peak potential (Fig. 2c). However, considering the picture of the peaks, they are still very well defined (for the TC–CTAB mixture), there is no drastic change in width at half their height. This indicates the enhanced dynamics of Bi(III) ions electro-reduction acceleration due to the presence of the cationic surfactant and the formation of mixed adsorption layers at the R-AgLAFE/chlorate(VII) interface (Martyna et al. 2022). On the other hand, the effect of mixed TC-SDS adsorption layers is the change of electrode process reversibility dynamics which is demonstrated by a better defined SWV peak and a decrease in its height, especially above the CMC (Kaliszczak and Nosal-Wiercińska 2018) (Fig. 2d).
a SWV peaks of 1·10−3 mol·dm−3 Bi(III) ions electro-reduction with the influence of CTAB addition; b SWV peaks of 1·10−3 mol·dm−3 Bi(III) ions electro-reduction with the influence of SDS addition; c SWV peaks of 1·10−3 mol·dm−3 Bi(III) ions electro-reduction at the presence of 1·10−3 mol·dm−3 2-thiocytosine and with the influence of CTAB addition; d SWV peaks of 1·10−3 mol·dm−3 Bi(III) ions electro-reduction at the presence of 1·10−3 mol·dm−3 2-thiocytosine and with the influence of SDS addition
From the CV voltammetograms (Fig. 3a, b), the values of the anodic and cathodic peak potential difference \(\Delta E\) were determined, which have simple relationships with the changes in the height and position of the corresponding peaks in the SWV square wave voltammetry. The presence of 1·10−3 mol dm−3 TC definitely increases the reversibility of the proces (Nosal-Wiercińska et al. 2021a). The \(\Delta E\) values decrease very much compared to those obtained for the basic electrolyte (1·10−3 mol dm−3 Bi(III) in 1 mol dm−3 chlorate(VII)).
The addition of surfactants to such a system affects the changes in \(\Delta E\). CTAB causes a further decrease in the potential difference of the anodic and cathodic peaks (Fig. 3a), while SDS has the opposite effect (Fig. 3b). These observations lead to the conclusion that the presence of mixed adsorption layers influences significantly the changes in the dynamics of acceleration of the electro-reduction process of Bi(III) ions by 2-thiocytosine (Nosal-Wiercińska et al. 2021a).
The studies carried out (lack of linearity of the real rate constants \({k}_{\mathrm{f}}\) determined by the impedance method, taking into account the influence of the double layer) on the Bi(III) ions electro-reduction as a function of the electrode potential (Nosal-Wiercińska 2014) confirmed the multi-step character of the Bi(III) ions electro-reduction process and in the presence of 2-thiocytosine and 2-thiocythosine–surfactant mixtures (Fig. 4a, b). On the other hand, small changes in the anodic and cathodic peak potential difference \(\Delta E\) along with the change in the polarization rate (Table 1a, b) for all the studied systems indicated that the rate of Bi(III) ion electro-reduction process was controlled by the chemical reaction.
a Dependence of 1·10−3 mol·dm−3 Bi(III) electro-reduction rate constants at the presence of 1·10−3 mol·dm−3 of 2-thiocytosine and various concentrations of CTAB indicated in the figure as a function of R-AgLAFE electrode potential; b Dependence of 1·10−3 mol·dm−3 Bi(III) electro-reduction rate constants at the presence of 1·10−3 mol·dm−3 of 2-thiocytosine and various concentrations of SDS indicated in the figure as a function of R-AgLAFE electrode potential
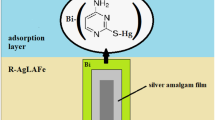
The previous studies in the case of 2-thiocytosine indicate a reaction of the formation the Bi–(RS–Hg) active complexes on the electrode surface, which mediate electron transfer (Nosal-Wiercińska et al. 2021a). The Bi(III) ions electro-reduction process in 1 mol dm−3 chlorate(VII) in the presence of 2-thiocytosine occurs in the adsorption layer. No changes were observed in the mechanism of Bi(III) ion electro-reduction process in the presence of 2-thiocytosine-CTAB mixture in the base electrolyte solution. The differences \(\Delta E\) with the change in the polarization rate are small (at these low rates v) as for 2-thiocytosine alone. In the case of the mixed adsorption layer formation (Martyna et al. 2022), the adsorption layer can be unraveled due to the repulsive interaction between the positively charged nitrogen atoms in the surfactant molecule, directed with the hydrophilic end toward the solution (Nieszporek and Dagci 2014).
This will make it easier for the previously formed Bi–(RS–Hg) active complexes to get to the electrode surface, resulting in an increase in the rate of the Bi(III) ion electro-reduction process (Fig. 5). Whereas for the 2-thiocytosine-SDS system, there is observed different dependence, the significant differences \(\Delta E\) with the change of polarization rate especially above CMC (5·10−5 mol·dm−3) (Martyna et al. 2022) indicate the changes in the mechanism of Bi(III) ions electro-reduction process. Most probably, the surfactant molecules block the electrode surface pushing out the previously formed Bi–(RS–Hg) active complexes from the adsorption layer (Fig. 6).
This changes the catalytic dynamics of 2-thiocytosine towards inhibition. A similar effect was observed in the paper (Kaliszczak and Nosal-Wiercińska 2018, 2019, 2020). However, it should be emphasized that in both cases the Bi–(RS–Hg) complex plays a key role, as it is the 2-thiocytosine which dominates in the formation of adsorption equilibria of the studied mixed adsorption layers (Martyna et al. 2022).
Kinetic parameters
The determined cathodic transition coefficients (\(\mathrm{\alpha }\)), standard rate constants (\({k}_{s}\)) based on the CV cyclic voltammetry curves indicated quantitatively changes in the catalytic effect of 2-thiocytosine in relation to the presence of surfactants in the basic electrolyte solution (Tables 2, 3).
The increase in the values of the transition coefficients \(\mathrm{\alpha }\) after the addition of CTAB into the base electrolyte solution containing a constant concentration of 2-thiocytosine indicates an increase in the reversibility of the Bi(III) ion electro-reduction process. This also translates into an increase in the standard rate constants \({k}_{\mathrm{s}}\), confirming the increased dynamics of TC catalysis in the presence of CTAB.
However, the addition of SDS to the tested system shows a decreasing trend in \(\mathrm{\alpha }\). The values of rate constants decrease as the concentration of SDS in the chlorate(VII) solution containing 1·10−3 mol·dm−3 2-thiocytosine increases. Especially the decreasing tendency is visible for the concentration 5·10−5 mol·dm−3 SDS (CMC value) and above. This confirms changes in the electrode mechanics and more blocking of the electrode surface due to the increasing surfactant concentration in the base electrolyte solution (Kaliszczak and Nosal-Wiercińska 2018).
Conclusion
The presence of mixed TC-CTAB and TC-SDS adsorption layers influences on the changes of dynamics of acceleration of the Bi(III) ions electro-reduction process by 2-thiocytosine. The main role of the Bi–(RS–Hg) complex, in relation to the dominance of 2-thiocytosine in the formation of adsorption equilibria of the studied mixtures, was pointed out. As the concentration of CTAB increases in the electrolyte solution in the presence of substances that accelerate the depolarization electro-reduction, the reversibility of the electrode process increases. As a result of the unsealing of the mixed adsorption layer, the Bi–(RS–Hg) active complexes formed earlier have a better access to the electrode, which explains the increase in the rate of the electrode process. On the other hand, in the case of the 2-thiocythosine-SDS mixture, the opposite effect is observed. The surfactant molecules, in the form of hemimicelles, block the electrode surface pushing out from the adsorption layer the Bi–(RS–Hg) active complexes formed earlier, which leads to a change in the mechanism and consequently, to a decrease in the kinetics of the Bi(III) ions electro-reduction process. However, it should be noted that the dehydration and active complex steps in the multi-step electro-reduction process of Bi(III) ions are much faster than the electron transition stages. This prevents detection of the active complexes formation on the electrode surface and their composition. The role of variety in the structure of these complexes but in the combination of Bi-H2O-2-thiocytosine appears to be important.
The results of the proposed research can point in a certain direction in medicine to identify and know other somewhat mechanisms of controlled drug release. There is a need to study these mechanisms of drug action and search for new systems of controlled drug release what can be used to monitor the patient's health.
References
Dalmata G (2005) Kinetics and mechanism of Zn (II) ions electroreduction catalyzed by organic compounds. Electroanalysis 17:789–793
Fouda AS, Attia AM, Rashed AM (2017) Corrosion inhibition of mild steel in aqueous solutions using nonionic surfactants. Protect Mat Phys Chem Surf 53:743–752
Ikeda O, Watanabe K, Taniguchi Y, Tamura H (1984) Adsorption effects of highly polarizable organic compounds on electrode kinetics. Bull Chem Soc Jpn 57:3363–3367
Kaliszczak W, Nosal-Wiercińska A (2018) The importance of the active complexes of 6-mercaptopurine with Bi(III) with regards to kinetics and electrode mechanism changes in the presence of non-ionic surfactants. J Electroanal Chem 828:108–115
Kaliszczak W, Nosal-Wiercińska A (2019) Influence of mixed 6-thioguanine-nonionic surfactant adsorption layers on kinetics and mechanism of Bi(III) ion electroreduction. Electrocatalysis 10:621–627
Kaliszczak W, Nosal-Wiercińska A (2020) Change in the dynamics of the catalytic action of azathioprine on the electroreduction process of Bi(III) ions under the influence of surfactants in the context of controlled drug release. J Electroanal Chem 862:114033
Koundal M, Singh AK, Sharma C (2022) Study on the effect of imidazolium ionic liquid as a modulator of corrosion inhibition of anionic surfactant sodiumdodecylsulfate (SDS) on mild steel in sodium chloride solution. J Mol Liq 350:118561
Martyna M, Grochowski M, Urban T, Nosal-Wiercińska A (2022) Study of 2–thiocytosine/surfactant adsorption at the R-AgLAFe/chlorate(VII) interface—impact of surfaktant ionic charakter. Physicochem Probl Miner Process 58(2):144322
Nieszporek J (2011) Influence of sodium 1-decanesulfonate on the enthalpy of activation of two-step Zn2+ ions electroreduction. J Electro Anal Chem 662:407–414
Nieszporek J, Dagci K (2014) Influence of N-octanoyl-N-methylglucamine and N-decanoyl-N-methylglucamine on the kinetics and mechanism of Zn2+ ions electroreduction. Electrochim Acta 125:473–481
Nosal-Wiercińska A (2010a) Catalytic activity of thiourea and its selected derivatives on electroreduction of In(III) in chlorates (VII). Centr Eur J Chem 8:1–11
Nosal-Wiercińska A (2010b) The kinetics and mechanism of the electroreduction of Bi(III) ions from chlorates (VII) with varied water activity. Electrochim Acta 55:5917–5921
Nosal-Wiercińska A (2014) Inter-molecular interactions in systems containing Bi(III)–ClO4–H2O-selected amino acids in the aspect of catalysis of Bi(III) electro-reduction. Electroanalysis 26:1013–1023
Nosal-Wiercińska A, Kaliszczak W (2021) Role of active complexes of selected thiopurine derivatives with Bi(III) ions related to kinetics and mechanism of the electrode process in the surfactant presence. Springer Proc Phys 243:523–538
Nosal-Wiercińska A, Kaliszczak W, Grochowski M, Wiśniewska M, Klepka T (2018) Effects of mixed adsorption layers of 6-mercaptopurine–Triton X-100 and 6-mercaptopurine–Tween 80 on the double layer parameters at the mercury/chlorates(VII) interface. J Molec Liq 253:143–148
Nosal-Wiercińska A, Martyna M, Skrzypek S, Szabelska A, Wiśniewska M (2021a) Electroreduction of Bi(III) ions in the aspect of expanding the “cap-pair” effect: the role of the nanosized active complexes. Appl Nanosci 1–9
Nosal-Wiercińska A, Martyna M, Grochowski M, Baś B (2021b) First electrochemical studies on “CAP—PAIR” effect for BI(III) ion electroreduction in the presence of 2-thiocytosine on novel cyclically renewable liquid silver amalgam film electrode (R-AgLAFE). J Electrochem Soc 168:066504
Refay HM, Badawy N, Hamada A, Hassen ES (2022) Separation of some anionic dyes using reverse micelles of CTAB and SDS as efficient surfactants adsorbents from aqueous medium. Int J Chem Eng 7484479
Saba J, Nieszporek J, Gugala D, Sienko D, Szaran J (2013) Influence of the mixed adsorption layer of 1-butanol/toluidine isomers on the two step electroreduction of zinc(II) ions. Electroanalysis 15:33–39
Shahrokhian S, Amiri M, Amiri M (2004) Voltammetric determination of thiocytosine based on its electrocatalytic oxidation on the surface of carbon-paste electrode modified with cobalt Schiff base complexes. J Solid State Electrochem 11:1133–1138
Sluyters-Rehbach M, Sluyters JH, Yeager E, Bockris M, Conway BE, Sarangapani S (1984) Comprehensive treatise of electrochemistry. Plenum Press, New York
Souto RM, Sluyters-Rehbach M, Sluyters JH (1986) On the catalytic effect of thiourea on the electrochemical reduction of cadmium (II) ions at the DME from aqueous 1 M KF solutions. J Electroanal Chem 201:33–45
Funding
The work has been supported by the National Science Centre (Poland) under Grant No. DEC-2018/29/B/ST5/01572.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Nosal-Wiercińska, A., Martyna, M. & Wiśniewska, M. Influence of mixed 2-thiocytosine–ionic surfactants adsorption layers on kinetics and mechanism of Bi(III) ions electro reduction: use of the nanostructured R-AgLAFE. Appl Nanosci 13, 4737–4745 (2023). https://doi.org/10.1007/s13204-022-02605-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13204-022-02605-4