Abstract
The paper discusses the electroreduction of Bi(III) ions in the aspect of expanding the “cap-pair” effect.
The “cap-pair” rule is associated with the acceleration of the electrode’s processes by organic substances. The interpretation of the “cap-pair” effect mechanism was expanded to include the effect of supporting electrolyte concentration on the acceleration process and the type of electrochemical active as well as used protonated organic substances. It has also been shown that the phenomena occurring at the electrode/solution interface can influence a change in the dynamics of the electrode’s process according to the “cap-pair” rule.
Similar content being viewed by others
Explore related subjects
Find the latest articles, discoveries, and news in related topics.Avoid common mistakes on your manuscript.
Introduction
Research into electrode processes taking place in aqueous solutions is one of the basic topics of modern electrochemistry due to its practical aspect. The most common examples are the use of lead batteries in cars, lithium-ion batteries in cell phones, control and prevention of corrosion, which is decisive for the life and reliability of many devices or sensors used in monitoring environmental pollution. Besides these examples, there are many other areas of human activity using from electrochemical engineering applications, such as bioelectronics, electrochemical waste disposal or electrochemical synthesis of oxidants. The use of electrochemical methods is also observed in diagnostics and medical therapy, e.g. when determining and searching for new drugs or biomaterials.
The commonly used electrochemical techniques have many advantages, among which we can distinguish: relatively low cost of equipment, ease of miniaturisation and automation, high sensitivity, precision, accuracy, and simplicity of measurement.
It should be noted that in the extensive spectrum of analytical techniques, few methods satisfy all these criteria. Electrochemical measurement techniques such as square Wave Voltamperometry (SWV), Pulse Differential Voltamperometry (DPV), Square Wave Stripping Voltamperometry (SWAdSV) or Cyclic Voltamperometry (CV) are commonly used to determine depolarisers and biologically active compounds as well as to study electrode mechanisms (Galus 1977, 1979; Skrzypek et al. 2011, 2012; Mirceski et al. 2005). At present, one of the fastest developing techniques is electrochemical impedance spectroscopy (EIS), which allows for determining the mechanisms of processes taking place at the electrode/solution interface or to investigate the applicability of new electrode materials (Lasia 1999).
A very important stage of electrochemical research is the choice of a suitable working electrode. Carbon electrodes are commonly used, the most popular are glass carbon electrodes (GCE) (McCreery 2008; Ghaani et al. 2018), carbon paste electrode (CPE) (Asadollahzadeh et al. 2017), boron-doped diamond electrode (BDDE) (Baluchova et al. 2019) or carbon printed electrodes (SPE) (Casella et al. 2016; Raza 2018). These are solid electrodes, which due to processes taking place on their surface, both during their preparation and use and a lack of reproducibility of the surface during mechanical cleaning are, however, characterised by worse reproducibility than mercury electrodes, with a constant change of parameters (range of useful potentials, residual current) and low stability. As a result, their practical application in determining electrode mechanisms is very limited.
Mercury as an electrode material, despite its defects, also has several important advantages (Kalvoda 2007; Barek 2013; Vaškelis 2005), such as:
- neutrality to most electrolytes,
- high hydrogen overvoltage,
- ease of amalgam formation with most metals,
- exceptional smoothness and good surface definition.
At present, the electrode with the highest coefficient of repeatability, precision and surface reproducibility is the electrode with controlled growth of the surface of the drops (Controlled Growth Mercury Drop Electrode—CGMDE), developed by Kowalski (Kowalski et al. 1987) and introduced into ongoing production by companies such as Bioanalytical Systems (BAS), USA; Methrom, Poland or MTM-ANKO Kraków, Poland.
The type of investigation determines the choice of base electrolyte. The weak complex-forming properties of ClO4‾ ions, the tendency to destruct the water structure, and the fact that chlorate ions adsorb to a small extent on the mercury surface indicated the choice of chlorate(VII) solution as the basic electrolyte (Nosal-Wiercińska 2010).
Gaining knowledge into the influence that organic substances have on the rate of electrode reactions is of great analytical importance as well as in developing technological and pharmacological characteristics. Organic substances may inhibit, accelerate, or not affect the electrode’s process.
The conditions under which an organic substance acts as a catalyst were formalised in 1978 by the Sykut team defining them as a “cap-pair” rule (Sykut et al. 1978). It follows that the non-active electrochemical organic substance molecule must contain sulphur or nitrogen atoms with free electron pairs that are able to form coordination bonds with the depolariser ions, while the depolariser redox potential must be within the labile equilibrium for adsorption of the organic substance with the working electrode surface. The mechanism for the catalytic effect of metal cations electroreduction under “cap-pair” conditions, includes both chemical reactions and heterogeneous processes of charge transfer between the electroactive particle of the complex and the depolarised working electrode. The formation of electroactive complexes with depolarisers is possible both in the adsorption layer for zinc(II) ions (Dalmata et al. 2004; Dalmata 2005; Saba et al. 2003), cadmium (Souto et al. 1986) indium(III) (Nosal–Wiercińska et al. 2002; Nosal–Wiercińska 2010) and bismuth(III) (Sykut et al. 1998; Komorsky–Lovrič et al. 1993; Nosal-Wiercińska 2010, 2013, 2014; Grochowski et al. 2016), and outside the layer for europium(III) ions (Ikeda et al. 1984). The goal of this current paper was to collect and summarise studies on the kinetics and mechanism of action for selected organic substances on the electroreduction of Bi(III) ions in chlorate(VII) solutions in terms of expanding the interpretation of the “cap-pair” effect mechanism.
It was pointed out:
- Effect of changes of water activity and used active (cysteine (CE) and cystine (CY)) and non-active (methionine (MT)) electrochemically accelerating organic substances on the mechanism and kinetics of Bi(III) ion electroreduction,
- Influence of protonation changes of selected amino acids (homocysteine (HCE), homocystine (HCY) and ethionine (ET)) on the mechanism and kinetics of Bi(III) ion electroreduction,
- The effect of the mixed adsorption layers forming (6–mercaptopurine (6MT)–surfactant, 6-thioguanine (6TG)–surfactant and azathioprine (AZA)–surfactant) on the mechanism and kinetics of Bi(III) ion electroreduction.
The practical aspect of these studies is related to the possibility of directing and indicating new ways of determining depolarisers as well as substances that may cause a disturbance of homeostasis in a living organism. This can help determine the complex mechanism of action for certain drugs in the body to help monitor a patient's health.
Results and discussion
Kinetics and mechanism of Bi(III) ion electroreduction process in the presence of selected amino acids in chlorate(VII) solutions with different water activity
The influence of both water activity and selected amino acids on the kinetics and mechanism of the Bi(III) ion electroreduction process in 1–8 mol·dm−3 chlorates(VII) was demonstrated.
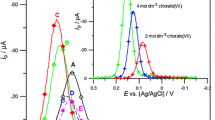
The presence of methionine (Nosal-Wiercińska 2011a, b), cysteine (Nosal-Wiercińska 2011a, b) or cystine (Nosal-Wiercińska 2011a, b; Nosal-Wiercińska 2012) in the supporting electrolyte solution causes both an increase of the slope of the polarographic wave and the SWV current of the Bi(III) ion electroreduction peaks, which indicates an increase in the reversibility of the Bi(III) ion electroreduction process (Dalmata 2005). Such observations also result from the course of CV voltammetric curves. Small changes in the distance between the anodic and cathodic peak potentials ΔE(a-c) along with a change in the polarisation rate indicated that the process of the electroreduction of Bi(III) ions in 1—8 mol·dm−3 chlorate(VII) in the presence of the amino acids under study is controlled by the kinetics of the reaction preceding the transition of electrons (Dalmata 2005; Fijałkowska et al. 2020). The acceleration of electrode processes only by organic substances that have free electron pairs of sulphur or nitrogen atoms, suggests the formation of complexes under specific conditions that exist on the electrode’s surface. The obtained results indicate that in the case of methionine a Bi–methionine complex is formed on the electrode’s surface. In the case of cysteine and cystine, respectively, mercury cysteine thiolates (I) and (II) (Heyrovský et al. 1994, 1999), which can form an active complex from Bi(III), are adsorbed for mercury. Therefore, cysteine and cystine are a bridge in the formation of these complexes. It has been found that Bi(III) reacts with mercury (II) cysteine thiolate Hg(SR)2 (Nosal-Wiercińska 2011a, b). The real rate constants kf (taking into account the influence of the double layer) of the Bi(III) ion electroreduction as a function of the electrode’s potential determined from impedance measurements (Nosal-Wiercińska 2011a, b) indicated that also in the presence of substances catalysing the Bi(III) ion, the electroreduction process is carried out in stages. In addition, the effect of catalysts on the transition of the first electron is usually much more significant than on the transition of the other electrons. This is evidence that Bi(III) ion complexes with the accelerating substance are formed already before the first electron passes, which is the slowest stage and determines the speed of the whole process. The nature of changes lnkf = f(E) (Nosal-Wiercińska 2011a, b) in chlorate(VII) solutions in the presence of all studied amino acids show the differences in the mechanism of electroreduction process in solutions with high water activity compared to solutions with low water activity. The determined standard rates constants ks (Nosal-Wiercińska 2011a, b, 2012) indicated that the catalytic action of amino acids increases in the cystine < methionine < cysteine series—for chlorates(VII) with high water activity. For higher chlorates(VII) concentrations (from 5 to 8 mol·dm−3), a comparable effect of amino acids on the Bi(III) ion electroreduction rate is observed (Fig. 1).
Effects of protonation of some amino acids on the kinetics and mechanism of Bi(III) ions electroreduction
Significant differences in the kinetics and mechanism of the Bi(III) ion electroreduction process were observed due to the change of HClO4:NaClO4 ratio in 2—6 mol·dm−3 chlorate(VII) solutions (Nosal-Wiercińska et al. 2011) and the presence of homocysteine, homocystine or ethionine (Grochowski et al. 2016, 2017; Nosal-Wiercińska et al. 2017).
The increase in SWV peak current of the Bi(III) ion electroreduction along with a simultaneous decrease in the peak width at half of its height, indicates an increase in the reversibility of the Bi(III) ion electroreduction process in the presence of the studied amino acids (Grochowski et al. 2016, 2017; Nosal-Wiercińska et al. 2017). The magnitude of this effect depends on the concentration of amino acids and changes in the ratio of HClO4 and NaClO4 in solutions of chlorates(VII) with different water activity (Grochowski et al. 2016, 2017; Nosal-Wiercińska et al. 2017).
Confirmation of such changes in the reversibility of the electrode’s process is a clear reduction in the distance between the anode and cathode peaks taken from the cyclic voltammetry curves.
The investigations indicated a multistage character of the electrode’s process also in the presence of HCE, HCY and ET, control of the rate of Bi(III) ion electroreduction process by a chemical reaction (Dalmata 2005). It was indicated that active complexes are formed on the electrode’s surface, which mediates electron transfer. It should also be noted that due to the mentioned electrochemical reactivity of both homocysteine and homocystine, which react with mercury in the same way as cysteine and cystine (Galík et al. 2010), we can discuss Bi—Hg2(SR)2 or Bi—Hg(SR)2 complexes. According to the literature reports (Nosal-Wiercińska 2011a, b), Bi(III) reacts with mercury cysteine thiolate—Hg(SR)2. This form of anodic mercury oxidation in the presence of homocysteine or homocystine is adsorbed in the range of Bi(III) reduction potentials (~ 0 mV) and is loosely bonded to the electrode’s surface (Heyrovský et al. 1994, 1999). However, in the case of polarographically non-active ethionine, complexes of type Bi—ethionine are formed on the electrode’s surface (Galík et al. 2010; Read et al. 2004).
The real rate constants kf of the Bi(III) ion electroreduction as a function of the electrode potential that was determined from the impedance measurements (Nosal-Wiercińska 2011a, b) indicate differences in the mechanism of the electroreduction process in solutions differing in the degree of amino acid protonation. The correlation of kinetic parameters shows that both the water activity and the presence of amino acids to a different degree of protonated amino acids affect the rate of electroreduction of Bi(III) ions. The ks values indicate that the catalytic action of amino acids increases in the ET < HCY < HCE series for chlorates(VII) with a high water activity of 2 mol∙dm−3. For 4 and 6 mol∙dm−3 chlorates(VII), a comparable effect of the amino acids studied on the rate of the Bi(III) ion electroreduction is observed, especially for HCE and HCY. However, the catalytic action of ethionine is slightly higher compared to cysteine and cystine derivatives (Nosal – Wiercińska et al. 2017; Read et al. 2004) (Fig. 2a–c). As the quantity of NaClO4 in the base electrolyte solution increases, the amino acid catalytic activity increases. On the other hand, an increase in the quantity of HClO4 in chlorate(VII) solutions causes much smaller changes in the kinetics of the Bi(III) ion electroreduction process in the presence of homocysteine, homocystine and ethionine. The highest catalytic activity was observed in 2 mol∙dm−3 chlorates(VII) for the highest quantity of sodium salt of chloric acid(VII) (2C solution) (Nosal – Wiercińska et al. 2018).
Influence of mixed thiopurine derivatives-nonionic surfactant adsorption layers on kinetics and mechanism of Bi(III) ion electroreduction
The introduction of thiopurine derivatives (6-mercaptopurine, 6-thioguanine, azathioprine) to solutions of Bi(III) ions in 2 mol·dm−3 chlorates(VII) indicates an increase in the reversibility of Bi(III) electroreduction (Nosal-Wiercińska 2011a, b) (an increase in the SWV peak current for Bi(III) ion electroreduction as well as a simultaneous reduction in the width of SWV peaks at half their height (Kaliszczak et al. 2018, 2019, 2020). The effect of the studied surfactants (Tween 80 and Triton X – 100) on the figure of the SWV peaks Bi(III) ions electroreduction in 2 mol⋅dm−3 chlorates(VII) at a constant concentration of accelerating substance (1⋅10–3 mol⋅dm−3 6TG, 6MP, AZA) depends on a change in the electrode process reversibility towards inhibition (Kaliszczak et al. 2018, 2019, 2020).
Similar changes of the reversibility of Bi(III) electroreduction due to the presence of thiopurine derivatives or surfactants are indicated by the CV voltammograms. The values ∆Ea-c decrease compared to those obtained for the basic electrolyte (1⋅10–3 mol⋅dm−3 Bi(III) in 2 mol⋅dm−3 chlorates(VII)). Therefore, the electrode process becomes faster. This is particularly noticeable with 6TG and 6MP. The addition of surfactants to such a system affects ∆Ea-c increase. There is a change in the dynamics of the catalytic action of thiopurine derivatives (Kaliszczak et al. 2018, 2019, 2020).
The study pointed to the control of the multistage Bi(III) ion electroreduction process by the reaction of formation of active complexes Bi–thiopurine on the electrode’s surface, certainly localised inside the adsorption layer which mediate in the transfer of electrons (Dalmata 2005; Nosal-Wiercińska 2011a, b). The adsorption of 6TG, 6MP, AZA (Nosal-Wiercińska et al. 2018a, b, 2019a; Kaliszczak et al. 2019; Szewczuk-Karpisz et al. 2020; Wiśniewska et al. 2020) should be mentioned, which will not limit the electrode’s surface but will favourably shift the balance of these complexes.
However, changes in the mechanism of the Bi(III) ion electroreduction process in the presence of a mixture of thiopurine derivatives and surfactants in the base electrolyte solution were associated with the blocking of the electrode’s surface by surfactants, which pushes the previously formed active complexes of Bi—thiopurine from the adsorption layer. The main role of Bi—thiopurine complexes has been indicated (Kaliszczak et al. 2018, 2019, 2020).
The kinetic parameter ks determined using of electrochemical techniques indicating the catalytic effect of thiopurine derivatives and changes in its magnitude in connection with the presence of 6TG–TritonX-100 and 6TG–Tween 80, 6MP–TritonX-100 and 6MP–Tween 80 or AZA–TritonX-100 and AZA–Tween 80 mixtures (Fig. 3a, b) (Kaliszczak et al. 2018, 2019, 2020).
The electrode’s mechanism
The above considerations and literature data (Dalmata 2005) suggest the following mechanism of the catalytic action of organic substances on the Bi(III) ion electroreduction in a solution with non-complexing properties, including transition stages:
- Partial dehydration of Bi(III) ions and the formation of the active complex (I)
- First electron transfer
- Further dehydration and the formation of the active complex (II)
- Second electron transfer
- Dehydration of Bi(III) ions and the formation of the active complex (III)
- Third electron transfer and amalgam formation
where active complex: Bi – substance catalysing or Hg(SR)2 and a + b + c = 9 and a > b > c.
Materials and methods
Chemicals
All reagents: NaClO4, HClO4, Bi(NO3)3∙5H2O, amino acids such as: methionine, cysteine, cystine, ethionine, homocysteine, homocystine and thiopurine derivatives such as: 6-mercaptopurine, 6-thioguanine, azathioprine and Triton X-100 and Tween 80 (Fluka) were of analytical grade. The water applied to prepare all solutions was purified in the Millipore system.
The supporting electrolytes x mol·dm−3 NaClO4 + 1 mol·dm−3 HClO4 (where 0 ≤ x ≤ 7) or the 2, 4 and 6 mol∙dm−3 chlorate(VII) solutions of HClO4:NaClO4 concentration ratios: (1:1) solution A, (1:4) solution B, (1:9) solution C, (4:1) solution D, (9:1) solution E were examined (Nosal-Wiercińska et al. 2015). A solution of 1·10–3 mol·dm−3 Bi(III) in the chlorates(VII) was the supporting electrolyte.
Apparatus
The electrochemical measurements were performed with an Autolab Fra 2/ GPES (Version 4.9) frequency response analyser (Eco Chemie, Utrecht, Netherlands). A three-electrode system consisting of Ag/AgCl/3 M KCl electrode as a reference and a platinum wire as an auxiliary electrode, dropping or hanging mercury– electrode with a controlled increase rate and a constant drop surface (0.014740 cm2), as a working electrode (MTM Poland) was used.
The all electrochemical measurements were made in thermostated cells at 298 K.
Research on the mechanism of the electrode process was associated with a need to determine the kinetic parameters that were presented in paper (Nosal-Wiercińska 2010).
Conclusions
In conclusion, the interpretation of the “cap-pair” effect mechanism was extended with increased detail by the influence of the supporting electrolyte concentration on the acceleration process and the type of electrochemical active organic substances used. It was also pointed out that the dynamics of the catalytic process of the substance's action on the electrode process has changed while respecting the assumptions of the “cap-pair” rule.
The rate of multistage Bi(III) ion electroreduction process in chlorates(VII) with different water activity is influenced by both the presence of catalysts and their protonisation.
Systematic studies on the kinetics and electrode mechanisms have clearly indicated a multistage process and the main role of active complexes (Bi—accelerating substance (electrochemical non-active or electrochemical active), mediating in the electron transition, localised in the adsorption layer. It has been shown that the first chemical stage of formation of unstable complexes is the most important and determines the kinetics of the overall Bi(III) ion electroreduction process. Then, there is a partial loss of the hydration envelope by the Bi(III) ions, which change their electrostatic potential by locating near OHP. The absence of active complexes in solution (no dependence of the formal potential on the concentration of the organic substance) was demonstrated. Moreover, all studied organic substances adsorb on the electrode surface. The adsorption of the catalytic substance on the electrode does not limit its surface but additionally activates it by positively shifting the Bi(III) ion complexing equilibrium (confirmation of the important role of adsorption in the cap-pair effect mechanism). The varied structure and properties of active complexes probably determine the different catalytic activity. It should also be noted that active complexes dominate in creating the adsorption equilibrium despite a change in the dynamics of the catalytic action of the substance on the electrode’s process caused by blockage of the electrode’s surface by physically absorbed surfactants. Therefore, the assumptions of the "cap-pair" rule are respected.
References
Asadollahzadeh H, Ranjbar M (2017) Modified carbon paste electrode In2S3/CPE nanoparticles for electrochemical determination of oxalic acid by cyclic voltammetry. J Clust Scien 28:1273–1283
Baluchova H, Danhel A, Dejmkova H, Ostatna V, Fojta M, Schwarzova-Peckova K (2019) Recent progress in the applications of boron doped diamond electrodes in electroanalysis of organic compounds and biomolecules - a review. Anal Chim Acta 1077:30–66
Barek J (2013) Possibilities and limitations of mercury and mercury-based electrodes in practical electroanalysis of biologically active organic compounds. Port Electrochim Acta 31:291–295
Casella IG, Bonito R, Contursi M (2016) Determination of some β-Blockers by electrochemical detection on polycrstalline gold electrode after solid phase extraction (SPE). Electroanalysis 28:1060–1067
Dalmata G, Nosal Wiercińska A, Zięcina T (2004) The influence of diaminotoluene isomers on the two-step electroreduction of Zn(II) ions in acetate buffers at different acidities. Collect Czech Commun 69:267–278
Dalmata D (2005) Kinetics and mechanism of Zn(II) ions electroreduction catalyzed by organic compounds. Electroanalysis 17:789–793
Fijałkowska G, Szewczuk-Karpisz K, Wiśniewska M (2020a) Anionic polyacrylamide influence on the lead(II) ion accumulation in soil – the study on montmorillonite. J Environm Health Sci Eng 18:599–607
Fijałkowska G, Szewczuk-Karpisz K, Wiśniewska M (2020b) Polyacrylamide soil conditioners: the impact on nanostructured clay minerals’ aggregation and heavy metals’ circulation in the soil environment. Nanomater Nanocomp Nanostruc Surfaces Applic 246:111–127
Galík MF, Bănică G, Bănică A, Švancara I, Vytřas K (2010) Homocysteine voltammetry at a mercury electrode in the presence of nickel ions. Electroanalysis 22:1733–1736
Galus Z (1977) Theoretical basis of chemical electroanalysis. PWN, Warsaw ((in Polish))
Galus Z (1979) Electroanalytical methods of determination of physicochemical constants. PWN, Warsaw ((in Polish))
Ghaani M, Rovera C, Pucillo F, Ghaani MR, Olsson RT, Scampicchio M, Farris S (2018) Determination of 2,4-diaminotoluene by a bionanocomposite modified glassy carbon electrode. Sens Actuators B: Chem 277:477–483
Grochowski M, Nosal-Wiercińska A, Wiśniewska M, Szabelska A, Gołȩbiowska B (2016) The effects of homocysteine protonation on double layer parameters at the electrode/chlorates(VII) interface, as well as the kinetics and the mechanism of Bi(III) ion electroreduction. Electrochim Acta 207:48–57
Grochowski M, Nosal – Wiercińska A. (2017) The influence of homocystine protonation on her catalytic activity in the process of electroreduction of Bi(III) ions in chlorates(VII). J Electroanal Chem 788:198–202
Heyrovský M, Mader P, Veselá V, Fedurco M (1994) The reactions of cystine at mercury electrodes. J Electroanal Chem 369:53–70
Heyrovský M, Vavřička S (1999) Electrochemical reactivity of homocysteine at mercury electrodes as compared with cysteine. Bioelectrochem Bioenerget 48:43–51
Ikeda O, Watanabe K, Taniguchi Y, Tamura H (1984) Adsorption effect of highly polarizable organic compounds on electrode kinetics. Bull Chem Soc Jpn 57:3363–3367
Kaliszczak W, Nosal – Wiercińska A. (2018) The importance of the active complexes of 6 - mercaptopurine with Bi(III) with regards to kinetics and electrode mechanism changes in the presence of non-ionic surfactants. J Electroanal Chem 828:108–115
Kaliszczak W (2019) Influence of mixed 6-thioguanine-nonionic surfactant adsorption layers on kinetics and mechanism of Bi(III) ion electroreduction. Electrocatalysis 10:621–627
Kaliszczak W, Grochowski M, Nosal-Wiercińska A, Brycht M, Checinska-Majak D, Gołębiowska B (2019) Effect of azathioprine on the parameters of double Hg/Chlorate(VII) interface layer in the presence of nonionic surfactants. Physicochem Probl Miner Process 55:1350–1356
Kaliszczak W, Nosal-Wiercińska A (2020) Change in the dynamics of the catalytic action of azathioprine on the electroreduction process of Bi(III) ions under the influence of surfactants in the context of controlled drug release. J Electroanal Chem 862:114033
Kalvoda R (2007) Is Polarography Still Attractive? Chem Anal 52:869–873
Komorsky-Lovrič S, Lovrič M, Branica M (1993) Effect of ionic strength on bi(iii) reduction from perchlorate medium. J. Electrochem. Soc. 140:1850–1853
Kowalski Z, Wong KH, Osteryoung RA, Osteryoung J (1987) Controlled-growth mercury drop electrode. Anal Chem 59:2216–2218
Lasia A (1999) Electrochemical Impedance Spectroscopy and Its Application: Conway, B.E.; Bockris, J.; White, R.E.: Modern Aspects of Electrochemistry, Kluwer Academic/Plenum Publishers
McCreery RL (2008) advanced carbon electrode materials for molecular electrochemistry. Chem Rev 108:2646–2687
Mirceski V, Skrzypek S, Ciesielski W, Sokołowski A (2005) Theoretical and experimental study of the catalytic hydrogen evolution reaction in the presence of an adsorbed catalyst by means of square-wave voltammetry. J Electroanal Chem 585:97–104
Nosal-Wiercińska A, Dalmata G (2002) Studies of the Effect of Thiourea on the Electroreduction of In(III) Ions in Perchloric Acid. Electroanalysis 14:1275–1280
Nosal-Wiercińska A (2010) Catalytic activity of thiourea and its selected derivatives on electroreduction of In(III) in chlorates(VII). Cent Europ J Chem 8:1–11
Nosal-Wiercińska A (2010) The kinetics and mechanism of the electroreduction of Bi(III) ions from chlorates(VII) with varied water activity. Electrochim Acta 55:5917–5921
Nosal-Wiercińska A (2011a) The catalytic influence of methionine on the electroreduction of Bi(III) ions in chlorates(VII) solutions with varied water activity. J Electroanal Chem 654:66–71
Nosal-Wiercińska A (2011b) The catalytic activity of cysteine and cystine on the electroreduction of Bi(III) ions. J Electroanal Chem 662:298–205
Nosal-Wiercińska A (2012) Electrochemical and thermodynamic study of the electroreduction of Bi(III) ions in the presence of cystine in solutions of different water activity. J Electroanal Chem 681:103–108
Nosal-Wiercińska A (2013) The role active complexes in the multistep process of Bi(III) Ion electroreduction in chlorate(VII) solutions with varied water activity in the presence of cysteine. Electrochim Acta 92:397–403
Nosal-Wiercińska A (2014) Intermolecular interactions in systems containing Bi(III) – ClO4- - H2O – selected amino acids in the aspect of catalysis of Bi(III) electroreduction. Electroanalysis 26:1013–1023
Nosal-Wiercińska A, Grochowski M, Wiśniewska M, Tyszczuk-Rotko K, Skrzypek S, Brycht M, Guziejewski D (2015) The influence of protonation on the electroreduction of Bi(III) ions in chlorates(VII) solutions of different water activity. Electrocatalysis 6:315–321
Nosal-Wiercińska A, Grochowski M (2017) The catalytic impact of ethionine on the multi-step electroreduction of Bi(III) ions in chlorates(VII) solutions. Electrocatalysis 8:492–497
Nosal-Wiercińska A, Grochowski M, Wiśniewska M (2018a) Effects of amino acids protonation on double-layer parameters of the electrode/chlorates(VII) interface, as well as kinetics and mechanism of Bi(III) ion electroreduction in the aspect of the “Cap–Pair” effect. Springer Proc Phys 210:285–300
Nosal-Wiercińska A, Kaliszczak W, Grochowski M, Wiśniewska W, Klepka T (2018b) Effects of mixed adsorption layers of 6-mercaptopurine – Triton X-100 and 6-mercaptopurine – tween 80 on the Double layer parameters at the mercury/chlorates(VII) interface. J Molec Liq 253:143–148
NosalWiercińska A, Kaliszczak W, Drapsa A, Wiśniewska M, Yilmaz S, Yagmur S, Saglikoglu G (2019a) Impact of water activity on double layer parameters at the mercury/chlorates(VII) Interface in the presence of mixed adsorption layers of 6-mercaptopurine–triton X-100. Adsorption 25:819–824
Nosal-Wiercińska A, Kaliszczak W, Drapsa A, Grochowski M, Wiśniewska M, Klepka T (2019b) Influence of nonionic surfactants and water activity on to adsorption of 6-thioguanine at the mercury/chlorates(VII) interface. Adsorption 25:251–256
Raza W, Ahmad K (2018) A highly selective Fe@ZnO modified disposable screen printed electrode based non-enzymatic glucose sensor (SPE/Fe@ZnO). Mat Let 212:2031–2034
Read JF, MacCormick KJ, McBain AM (2004) The Kinetics and mechanism of the oxidation of DL – ethionine and thiourea by potassium ferrate. Transition Met Chem 29:149–158
Saba J, Nieszporek J, Gugala D, Sienko D, Szaran J (2003) Influence of the mixed adsorption layer of 1-butanol/toluidine isomers on the two step electroreduction of zinc(II) ions. Electroanalysis 15:33–39
Skrzypek S, Mirceski V, Smarzewska S, Guziejewski D, Ciesielski W (2011) Voltammetric study of 2-guanidinobenzimidazole: electrode mechanism and determination at mercury electrode. Collect Czech Chem Commun 76:1699–1715
Skrzypek S (2012) Electrode mechanism and voltammetric determination of selected guanidino compounds. Cent Eur J Chem 10(4):977–988
Souto RM, Sluyters-Rehbach M, Sluyters JH (1986) On the catalytic effect of thiourea on the electrochemical reduction of cadmium(II) ions at the DME from aqueous 1 M KF solutions. J. Electroanal. Chem. 201:33–45
Sykut K, Dalmata G, Nowicka B, Saba J (1978) Acceleration of Electrode processes by organic compounds — “cap-pair” effect. J Electroanal Chem 90:299–302
Sykut K, Dalmata G, Nieszporek J (1998) The catalysis of the reduction of BiIII ions by methionine. Electroanalysis 10:458–461
Szewczuk-Karpisz K, Nowicki P, Sokołowska Z, Pietrzak R (2020) Hay-based activated biochars obtained using two different heating methods as effective low-cost sorbents: Solid surface characteristics, adsorptive properties and aggregation in the mixed Cu(II)/PAM system. Chemosphere 250:126312
Vaškelis A (2005) Requiem Poliarografijai Chemijos Institute. Chemija 16:58–66
Wiśniewska M, Fijałkowska G, Szewczuk-Karpisz K, Sternik D (2020) Aggregation and thermal properties of nanostructured montmorillonite covered with mixed adsorption layers of cationic polyacrylamide and hazardous lead(II) ions. Appl Nanosci 10:5499–5510
Funding
This research received no external funding.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Nosal-Wiercińska, A., Martyna, M., Skrzypek, S. et al. Electroreduction of Bi(III) ions in the aspect of expanding the “cap-pair” effect: the role of the nanosized active complexes. Appl Nanosci 12, 947–955 (2022). https://doi.org/10.1007/s13204-021-01758-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13204-021-01758-y