Abstract
Ceria-based solid solutions doped by Gd and/or Bi were synthesized by co-precipitation method from acid aqueous solution of cerium, gadolinium, and bismuth salts, followed by calcination at the temperature of 500 °C. Characterization of the synthesized samples by many methods was carried out. It is shown that all obtained powders of solid solutions crystallized into a cubic structure of the fluorite type, with an average particle size of 10–17 nm, and for the sample obtained through intermediate acetylacetonate complexes was 5–8 nm. The samples had a mesoporous structure with a pore diameter of 2–5 nm. These systems were tested as the catalysts in CO oxidation in the model gas mixture by the flow method. Gd0.05Bi0.15Ce0.8O2 system obtained through intermediate acetylacetonates has the highest activity, so this sample can be used as a catalyst.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In the context of significant interest in energy efficient technologies and environmental protection, materials based on nanostructured ceria are promising for solving existing problems. The advantage of such materials is due to that CeO2 has a large oxygen storage capacity (OSC) and high oxygen mobility, which can ensure its high catalytic activity and electrical conductivity (and, as a rule, these data for ceria correlate with explanations of dependencies). Thus, to realize an effective process, a crystalline structure with a sufficiently high concentration of oxygen defects is necessary, and an increase in the magnitude of OSC and the number of defects are possible by doping ceria with various oxides.
Among the previously proposed systems, Gd0.2Ce0.8O2 (GDC) electrolyte has high ionic conductivity, low activation energy, and good thermodynamic stability at 700–900 °C. Another way is proposed for the production of materials based on ceria and bismuth oxide, since δ-Bi2O3 has the highest ionic conductivity among all available oxides. As the content of bismuth oxide increases, the electrical conductivity also increases, but an additional tetragonal phase Bi2O3 in Bi x Ce1−x O2 (for x ≥ 0.2–0.3) also occurs, which affects the stability of such systems, and therefore the electrical conductivity and the catalytic activity in general (Bourja et al. 2011; Prekajski et al. 2013; Prekajski et al. 2014; Bourja et al. 2010). However, solid solutions of Gd–Bi–Ce–O were not previously proposed either as catalysts or as electrolytes. Thus, mesoporous nanopowders of Gd x Bi0.2−x Ce0.8O2 solid solutions are synthesized in this work, which has been tested as catalysts for the oxidation of carbon monoxide in a model gas mixture.
Materials and methods
Nitrates of cerium, bismuth, and gadolinium were used as the metal precursors. Appropriate amounts of salts were dissolved in concentrated nitric acid (total concentration of 0.667 M). After the dissolution, this mixture was added to distilled water (total concentration of 0.1 M); in one experiment after dissolution, acetylacetone was added. Then, the co-precipitation was carried out by the addition of aqueous ammonia up to pH 12. Ultrasonic processing was used during all process at 30 °C under stirring. The resulting precipitates were filtered, washed with distilled water-ethanol solution (H2O/C2H5OH = 9 vol.), dried at 150 °C for 12 h, and calcined in static air by heating at a rate of 4 °C/min from room temperature to 500 °C and kept at 500 °C for 1 h in a muffle furnace.
All powders were characterized by XRD (DRON-3M, CuKα radiation), nitrogen adsorption-desorption method (TriStar 3000 Micromeritics), TEM (Omega Leo-912AB), HR TEM (FEI Osiris) TG-DSC (Netzsch STA449F3), and FTIR (Nicolet AVATAR330). The elemental composition of the catalysts (pressed pellets for the better distribution determination) was measured by energy-dispersive X-ray spectroscopy (EDS) based on a scanning electron microscope TESCAN VEGA II SBU with an X-ray microanalysis system INCA Energy 300. The catalytic activity of the synthesized samples in the oxidation of CO was determined by the flow method at atmospheric pressure. The process was conducted in a U-shaped quartz reactor at the total flow rate of 60 ml/min within a temperature range of 20–700 °C. The temperature was measured with a thermocouple placed in the center of the catalytic bed. The catalyst sample mass was 0.3 g. The model gas mixture had the following composition, vol%: CO − 1; O2 − 2; N2 − balance. The concentrations of gases were determined by a gas chromatograph (Varian 450GC).
Results and discussion
The catalytic activity of synthesized nanopowders was determined in the model oxidation reaction of CO. Figure 1 shows as temperature increases the conversion of carbon monoxide increases, while the curve has an S-shaped type. The 100% oxidation temperature was following, °C: 620 (sample 1), 430 (sample 2), 404 (sample 2a), 472 (sample 3), 576 (sample 4), 593 (sample 5). Among them, the sample 2 had the lowest oxidation temperature, so the optimum catalyst composition was Gd0.05Bi0.15Ce0.8O2. Therefore, a similar system, but obtained with the addition of acetylacetone (sample 2a), was also tested, and the temperature of the complete CO conversion was reduced by 30 °C. When comparing the best catalyst Gd0.05Bi0.15Ce0.8O2 with other systems based on ceria [Zr0.12Y0.08Ce0.8O2, La0.12Y0.08Ce0.8O2, etc. (Mukherjee et al. 2016; Devaiah et al. 2015)] under close test conditions, the catalytic activity of the developed system was higher (the temperature of complete oxidation of CO is lower by 50–100 °C).
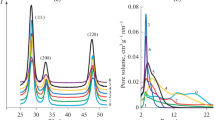
To understand the feature of this optimum catalyst composition (Gd0.05Bi0.15Ce0.8O2), the investigation of structure and morphological characterization was carried out. The obtained characteristics of synthesized nanopowders are presented in Table 1. According to XRD (Fig. 2a), samples have single-phase with fluorite structure (Fm3m, JCPDS-34-0394). Thus, in all cases the solid substitutional solution is formed in which the dopants enter the ceria lattice (Zagaynov 2015). The crystallite size, moreover, did not depend on Gd/Bi ratio and was about 15 nm. EDS analysis was used to characterize the composition of the best catalyst being described by Gd0.05–0.06Bi0.15–0.14Ce0.8O2 (Gd0.05Bi0.15Ce0.8O2—initial powder). It was shown earlier (Zagaynov and Konovalov 2017) that the preparation of solid solutions based on ceria through intermediate complexes, in particular acetylacetonates, was of interest, which was also realized in this work (sample 2a). Acetylacetone (Hacac) was added after dissolving the salts in nitric acid in an amount of Hacac/Σ(Me) = 1. Apparently, such system has a somewhat smaller lattice parameter than the sample without the use of Hacac (sample 2), which is associated with greater homogeneity of the resulting solid solution, and the crystallite size decreased.
The shape of the adsorption-desorption curves for all samples (Fig. 2b, presented only for samples 2 and 2a for example) corresponds to the IV type, which is typical for the formation of the mesoporous structure. It is shown that the value of the specific surface is somewhat higher for samples containing gadolinium than for those not doped with them (sample 1). It is also shown (Table 1) that when Hacac is added, the specific surface area decreased by half, which is characteristic for such synthesis method (Zagaynov and Konovalov 2017; Zagaynov and Buryak 2015). For all samples, the shape of the hysteresis loop corresponds to the H2 type: apparently, individual or connected cylindrical and bottle-shaped pores may be present, which is also confirmed by the pore size distribution (Fig. 2c). It was also noted that with the addition of Hacac, the pore size distribution formed by the small-angle particle boundaries decreased and the proportion of micropores decreased too (<2 nm, according to the t plot method).
Sample 2a has large aggregates of particles, according to the TEM data (Fig. 2d), the average particle size is 5–8 nm, which agrees well with the average crystallite size (8 nm) calculated by the Scherrer equation from XRD data. According to HR TEM (Fig. 2e), ceria nanoparticles have generally spherical–octahedral shapes with (111) plane, and no other particles except ceria solid solution were observed. It is known that the following parameters also influence the catalytic activity: the crystalline plane, the number of defined crystalline planes, the frequency of use of these planes, different numbers of the nearest bonded neighbors for Ce and O on the exposed surface, and, accordingly, the shape of ceria nanoparticles. This leads to the relative stability of the low index ceria surfaces following the trend: (111) > (110) > (100), but the formation energies of oxygen vacancies on different planes of ceria vary, following the order: (110) < (100) < (111), and the frequency of use of crystalline planes has the order: (100) < (110) < (111). So, the higher catalytic activity is due to the exposed (100) and (110) planes (form of particles: spindle-shaped, rods, cubes), but ceria nanoparticles preferentially expose stable (111) planes (Wu et al. 2012; Lawrence et al. 2011; Na et al. 2008; Mai et al. 2005; Lundberg et al. 2004; Sayle et al. 2002).
FTIR spectra (Fig. 2f) exhibit a broad band around 3200–3700 cm−1 and around 1630 cm−1 corresponding to vibration of OH group. Me–O band at 400–500 cm−1 attributed to the vibration of ceria-based solid solution. Samples also have residual nitrate and carbonate groups, which disappeared during the calcination; as seen there are no changes in the spectra before and after catalysis in sample 2a.
Thus, it was shown that a good distribution of the dopants in the ceria lattice can contribute to the formation of oxygen vacancies, thereby increasing the mobility of the lattice oxygen (Mukherjee et al. 2016); all these (as well as narrow pore size distribution) are the reasons for better catalytic activity of the materials; using Hacac intermediate acetylacetonate complexes lead to a more homogeneous structure with uniformly distributed oxygen vacancies.
Conclusions
The investigation showed that the expediency of using such catalysts in the oxidation of CO and solid solutions of Gd x Bi0.2−x Ce0.8O2 could be used as the catalyst or support for the subsequent deposition of an active phase, while the system Gd0.05Bi0.15Ce0.8O2, obtained by co-precipitation with simultaneous ultrasonic treatment through intermediate acetylacetonate complexes, and has the best catalytic activity in the reaction. As noted above, the catalytic activity and electrical conductivity for ceria-based materials correlate with each other: Gd0.05Bi0.15Ce0.8O2 (sample 2a) had the best electrical conductivity associated with the ionic conductivity of oxygen (Zagaynov et al. 2017).
References
Bourja L, Bakiz B, Benlhachemi A, Ezahri M, Villain S, Gavarri JR (2010) Synthesis and characterization of nanosized Ce1−x Bi x O2−δ solid solutions for catalytic applications. JTUSCI 4:1–8
Bourja L, Bakiz B, Benlhachemi A, Ezahri M, Villain S, Crosnier O, Favotto C, Gavarri J-R (2011) Structural, microstructural and surface properties of a specific CeO2–Bi2O3 multiphase system obtained at 600 °C. J Solid State Chem 184:608–614
Devaiah D, Tsuzuki T, Aniz CU, Reddy BM (2015) Enhanced CO and soot oxidation activity over Y-doped ceria–zirconia and ceria–lanthana solid solutions. Catal Lett 145:1206–1216
Lawrence NJ, Brewer JR, Wang L, Wu T-S, Wells-Kingsbury J, Ihrig MM, Wang G, Soo Y-L, Mei W-N, Cheung CL (2011) Defect engineering in cubic cerium oxide nanostructures for catalytic oxidation. Nano Lett 11:2666–2671
Lundberg M, Skårman B, Wallenberg LR (2004) Crystallography and porosity effects of CO conversion on mesoporous CeO2. Microporous Mesoporous Mater 69:187–195
Mai H-X, Sun L-D, Ya-W Zhang, Si R, Feng W, Zhang H-P, Liu H-C, Yan Ch-H (2005) Shape-selective synthesis and oxygen storage behavior of ceria nanopolyhedra, nanorods, and nanocubes. J Phys Chem B 109:24380–24385
Mukherjee D, Rao BG, Reddy BM (2016) CO and soot oxidation activity of doped ceria: influence of dopants. Appl Catal B 197:105–115
Na T, Milin Z, Juan L, Huaju L, Yong L, Wenjie S (2008) Facile synthesis of CeO2 nanospheres. Chin J Catal 29:1070–1072
Prekajski M, Fruth V, Andronescu C, Trandafilović LV, Pantić J, Kremenović A, Matović B (2013) Thermal stability of Ce1−x Bi x O2−δ (x = 0.1–0.5) solid solution. J Alloy Compd 578:26–31
Prekajski M, Stojmenović M, Radojković A, Branković G, Oraon H, Subasri R, Matović B (2014) Sintering and electrical properties of Ce1−x Bi x O2−δ solid solution. J Alloy Compd 617:563–568
Sayle DC, Maicaneanu SA, Watson GW (2002) Atomistic models for CeO2 (111), (110), and (100) nanoparticles, supported on yttrium-stabilized zirconia. J Am Chem Soc 124:11429–11439
Wu Z, Li M, Overbury SH (2012) On the structure dependence of CO oxidation over CeO2 nanocrystals with well-defined surface planes. J Catal 285:61–73
Zagaynov IV (2015) Sonochemical synthesis of mesoporous Gd x Zr y Ti z Ce1−x−y−z O2 solid solution. Ceram Int 41:8730–8734
Zagaynov IV, Buryak AK (2015) Mesoporous nanoscale ceria: synthesis from cerium (III) acetylacetonate and mechanism. J Sol–Gel Sci Technol 74:103–108
Zagaynov IV, Konovalov AA (2017) Influence of acetylaceton on mesoporous structure and catalytic activity of ceria-based solid solution. J Porous Mat 24:1247–1251
Zagaynov IV, Fedorov SV, Konovalov AA, Antonova OS (2017) Perspective ceria-based solid solutions Gd x Bi0.2−x Ce0.8O2. Mater Lett 203:9–12
Acknowledgements
This research was supported by Russian Science Foundation (No. 17-73-10331).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Zagaynov, I.V. Gd–Bi–Ce–O materials as catalysts in CO oxidation. Appl Nanosci 7, 871–874 (2017). https://doi.org/10.1007/s13204-017-0625-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13204-017-0625-4