Abstract
Electroless Ni–P films were deposited on mild steel substrate and sodium dihydrogen phosphite was used as a reducing agent. Temperature and pH of the bath were maintained at 90 °C and 6.86 to 10.86 respectively. The Ni–P film deposited samples were characterized by FE-SEM to know the surface and cross-section morphology of the film and the phases present were examined by X-ray diffraction. The chemical composition of the electroless Ni–P films was determined by EDS analysis. The thickness of the film was determined by FE-SEM cross-section image and was nearly 5 μm. The influence of pH values on film morphology was studied. Ni–P film was obtained in the pH ranges between 6.86 and 10.86 on mild steel substrate. Ni content in the film increased with increasing pH value of bath solution. Dense, uniform and small spherical shape grains were found in Ni–P film deposited at pH = 10.86. X-ray analysis shows a (222) preferential growth in all the films. The mean crystallite size of Ni–P films deposited at pH 6.86, 7.86, 8.86, 9.86, and 10.86, calculated by using the Scherrer-equation was found to be 44.7, 40.4, 41.2, 34.6 and 30.4 nm, respectively.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Ni–P alloy coatings have found numerous applications in many fields due to excellent properties of coatings, such as high corrosion resistance, high wear-resistant, good lubricity, high hardness and acceptable ductility (Gu et al. 2010; Lin and He 2005). Low-phosphorous deposits (1–3 % P) are crystalline and exhibit good wear resistance, but relatively poor corrosion resistance in a chloride environment. Medium-phosphorus deposits (5–8 % P) have a smaller crystalline size, whereas high-phosphorus deposits (more than 10 % P) exist mainly as a metallic glass. Medium-phosphorus deposits usually have properties lying between the low- and high-phosphorus deposits, and the solution offers enhanced deposition rates. High–phosphorus deposits typically exhibit the best corrosion properties, but suffer from slow rates of deposition (Martyak et al. 1993). Ni–P film finds extensive applications in different fields such as: (i) Aviation and Aerospace: Satellite and rocket components, rams pistons, valve components, (ii) Oil and Gas: Valve components, such as Balls, Gates, Plugs etc. And other components such as pumps, pipe fittings, packers, barrels, (iii) Chemical Processing: Heat exchangers, Filter units, pump housing and impellers, mixing blades, (iv) Textile: Printing cylinders, machine parts, spinnerets, threaded guides, (v) Automotive: Shock absorbers, heat sinks, gears, cylinders, brake pistons, and (vi) Food and pharmaceutical: Capsule machinery dies, chocolates molds, food processing machinery components. Due to its unique properties of excellent corrosion resistance, combined with a high wear resistance (Martyak et al. 1993).
In the present research work, nanostructured Ni–P film was developed by electroless deposition process. Electroless film deposition process involves the presence of a chemical reducing agent in solution to reduce metallic ions to the metal state. There are no external electrodes present, but there is electric current (charge transfer) involved. Instead of an anode, the metal is supplied by the metal salt; replenishment is achieved by adding either salt or an external loop with an anode of the corresponding metal that has higher efficiency than the cathode. Therefore, instead of a cathode to reduce the metal, a substrate serves as the cathode, while the electrons are provided by a reducing agent. The process takes place only on catalytic surfaces rather than throughout the solution (if the process is not properly controlled, the reduction can take place throughout the solution, possibly on particles of dust or of catalytic metals, with undesirable results). (Wei-Yu and Jenq-Gong 2004) reported Ni–P-based film by sputtering technique on 420 tool steel substrate in a dual-gun deposition system. (Timothy et al. 1998) reported the development of Ni–P film by laser process. The deposition of thin films by physical vapor deposition is complex process as it requires high vacuum and costly target materials. Also, size of the samples deposited is limited.
Merits of electroless deposition process are given below:
-
The process is simple, cheap, easy to handle and no sophisticated elements or instruments are required.
-
Good adherence between films and substrate can be obtained. Dopant addition can be easily controlled by altering composition of inorganic precursors. Also, there is flexibility of size and shape of the sample for deposition of the film by electroless.
Drawbacks of electroless process
Chemical bath cannot be re-used. Therefore, only required capacity of the chemical bath is prepared at a time, while in magnetron sputtering process, target can be re-used provided a central hole is not formed in the target. The aim of the present research work is to study the effect of pH (6.86–11.86) on microstructure of Ni–P electroless film on mild steel substrate. Ni–P film has excellent corrosion resistance in brine and acids environment, besides this alloy has increased wear resistance as desired in rotary or reciprocating service. The micro structural features of the Ni–P films were characterized by FE-SEM/EDS. XRD was used to identify the formation of different phases in Ni–P films.
Novelty of the electroless deposition process of the nanostructured film besides the simple, cheap, and the films can be deposited on any size of the sample. In aerospace, automobile and textile industries etc., size of the substrate is very large and therefore, it requires very large size of films. Another beauty of electroless film process is that it can be used for development of multilayer film at low temperature. The electroless deposition process used for fabricating multilayer film does not damage the already developed thin films beneath the top layer as it is a low temperature deposition technique (RT to 80–90 °C). On the other hand, if multilayer film is produced by any other physical vapor deposition process, dilution of the base layer films may occur due to relatively high deposition temperature in the sputtering technique. Therefore, electroless process is a potential and cost effective route to fabricate nanostructured Ni–P films.
Experimental procedure
Mild steel was chosen as substrate to deposit Ni–P film in the present work. Each specimen measuring approximately 15 mm (length) × 15 mm (width) × 5 mm (thickness) was cut from the mild steel plate and grounded by using SiC emery papers. Subsequently, it was polished on cloth polishing disc by using alumina powder followed by diamond paste. All samples were cleaned in acetone, ethanol and deionized water. Before deposition of Ni–P films, sensitization and activation of the samples were done by dipping into the 30 % hydrochloric acid for 2 min followed by water rinsing. Finally, clean and activated substrate was dipped into the prepared electroless bath at 90 °C for 2 h. The wet deposited samples were dried in air at room temperature. Samples were kept in the horizontal position to avoid draining and blending of the just deposited wet-films, which could lead to irregularities in the layer thickness and to surface inhomogeneties. The deposited films were annealed in a muffle furnace for 2 h in air environment at 200 °C temperature. The composition of the bath and the detailed process parameters used in electroless Ni–P films deposition are shown in Table 1. pH of the bath solution was maintained by ammonia solution. It was determined by portable digital laboratory pH meter model PX-176 (Panomex, Inc. India).
Characterization of the films
XRD (Bruker AXS, D8 Advance) measurements were made using CuKα radiation to characterize the electroless Ni–P films. The samples were scanned with a scan rate of 0.02o/s in the scan range of 20 to 80o. An average grain size of the Ni–P films is estimated using its XRD peak broadening according to Scherrer formula (Zou et al. 2008), as given in Eq. (1).
where D is the average size of crystallite, B is the broadening of the diffraction line measured at half maximum intensity, λ is the wavelength of the X-ray radiation (1.54052 Å, CuKα) and θ is the Bragg angle. The instrumental broadening has been considered for the calculation of grain size, and the value of 0.1 is subtracted (calculated using standard silicon sample) from the full-width half maximum (FWHM) value (B value).
FE-SEM/EDS (FEI, Quanta 200F) is used to characterize the microstructures and composition of the Ni–P films at an acceleration voltage of 20 kV.
Results and discussion
XRD analysis
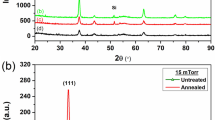
X-ray diffraction patterns were analyzed to get an idea of the influence of different pH of the bath of the deposited films on the crystal structure and the crystallite size of the Ni–P films. The structural properties of electroless Ni–P films with different pH (6.86–10.86) of the bath were investigated by XRD, and the results for all samples are shown in Fig. 1. XRD patterns of these samples exhibit peaks corresponding to (002), (222), (240), (611), (701) and (302) planes, which can be indexed as the Ni3P phase according to PCPDFWIN CAS Number: PDF# 340501. The (222) peak shows the highest intensity in all cases. There were no diffraction peaks detected for P-oxide secondary phases or other impurity phases within the sensitivity of our XRD measurements, implying that P ions were incorporated into the host Ni lattice, since the radius of P+2 ions (0.34 Å) is smaller than the Ni+2 ions (0.69 Å). The mean crystallite size of Ni–P films deposited at pH 6.86, 7.86, 8.86, 9.86, and 10.86, calculated by using the Scherrer-equation (Zou et al. 2008) was found to be 44.7, 40.4, 41.2, 34.6 and 30.4 nm, respectively.
FE-SEM/EDS analysis
The micrographs in Fig. 2a–e show the FE-SEM surface morphology of Ni–P films obtained from the solutions with different pH (6.86–10.86), we can observe an important effect of the pH on the film morphology. Figure 2a represents the microstructure of Ni–P film deposited at pH = 6.86 and large spherical shape grains were seen in the film. Figure 2b shows the plate type microstructure of Ni–P film deposited at pH = 7.86 and also found that deposited film has so many voids (Fig. 2b). By increasing the pH of the bath i.e. pH = 8.86, round and spherical shape grains were observed (Fig. 2c) in Ni–P film. It was also seen that films were deposited in layer by layer (Fig. 2c). Figure 2d represents the surface micrograph of Ni–P film deposited at pH = 9.86 and small spherical shape grains with dense morphology of the film were found. By further increasing the pH = 10.86 of the bath solution, microstructure of the film was more dense and very small spherical shape grains was observed (Fig. 2e). The cross-sectional FE-SEM microstructure of the Ni–P film deposited on mild steel substrate at pH = 10.86 was examined and is shown in Fig. 2f. The deposited Ni–P film has small spherical shape grains with dense microstructure. Cross-sectional FE-SEM micrograph (Fig. 2f) also revealed that deposited Ni–P film on mild substrate has two layer of the film. The thickness of the Ni–P film was calculated by its cross-sectional FE-SEM image, and it was found to be approximately 5 µm.
The detailed chemical compositions of the Ni–P films were analyzed by FE-SEM/EDS micrographs. Figure 3a shows EDS spectra obtained from surface FE-SEM/EDS images of Ni–P film and it confirms the presence of Ni and P in electroless Ni–P films. EDAX Genesis 32 software was used to calculate the composition of the Ni–P films, using elemental composition of the films obtained from EDS analysis. Figure 3b, c shows EDS analysis results obtained from surface FE-SEM/EDS images at pH = 6.86 (Fig. 3b) and pH = 10.86 (Fig. 3c). It was found that Ni and P content in Ni–P film at pH = 6.86 was 34.46 at. and 19.46 at. %, respectively. At pH = 10.86, Ni and P content in Ni–P film was determined 71.33 at. and 4.74 at. %. From EDS analysis, it was observed that increasing the pH of the bath solution, Ni content in Ni–P film was increasing and P content was decreasing. Thus EDS analysis revealed that phosphorus content in Ni–P film varied from 4 at. to 22 at. %. The Ni3P phase is observed in films at different pH values as evident from the XRD results and is also confirmed by Ni–P binary phase diagram.
It was observed from FE-SEM/EDS surface analysis that grain size of the Ni–P films was decreased by increasing the pH from 6.86 to 10.86. Uniform, dense and small spherical shape grains of Ni–P film deposited on mild steel substrate were observed at pH of 10.86 (Fig. 2e). Below pH = 6.86, Ni–P film was not uniformly covered the substrate, which results into dark appearance of the film. The reason might be less deposition of Ni favored. By increasing the pH of the bath, Ni content increases and P content decreases, it was confirmed by EDS analysis. Above pH = 10.86 the bath solution becomes strongly alkaline and two factors appear. One is that the concentration of free Ni+2 becomes low, another is that the resulting precipitation of basic salts in the bath consumes some Ni+2. Both of these actions finally stop deposition. Thus above 10.86 of pH, deposition of Ni–P film was not uniform. FE-SEM/EDS analysis gives two valuable information: (a) Uniformity in the micrograph of Ni–P film increased by increasing the Ni content and decreasing the P content in the film, (b) Grains size of the Ni–P film depend upon the amount of P content in the film. High P content of Ni–P film was appeared as large spherical shape grains (Fig. 2a) and low P content film was appeared as small spherical shape grains (Fig. 2e). Electroless Ni–P film deposited at pH = 10.86 has uniform, and dense film with small grain size (30.4 nm) can be used as decorative and functional coatings/films in electronics, machinery, automobile, and aerospace, because dense, uniform and small grain size film has unique properties of excellent corrosion resistance, combined with a high wear resistance (Martyak et al. 1993).
Conclusions
Electroless Ni–P film was deposited on mild steel substrate from sodium sulfate bath solution of pH ranges between 6.86 and 10.86 at 90 °C. Effect of bath pH on structure, composition, and morphology of the film was studied. EDS analysis of deposited Ni–P film showed the existence of nickel and phosphorus and the XRD pattern of the product confirmed the presence of these two elements in the coating in the form of Ni3P phase. Uniform, dense and small spherical shape microstructure of Ni–P film on mild steel substrate was obtained at a pH of 10.86. Ni content in Ni–P film increases with increase of pH value of bath solution.
References
Gu C, Lian J, Li G, Niu L, Jiang Z (2010) Electroless Ni-P plating on AZ91D magnesium alloy from A sulfate solution. J Alloys Compd 39:104–109
Lin CJ, He JL (2005) Cavitations erosion behavior of electroless nickel-plating on AISI1045 steel. Wear 259:154–159
Martyak N, Wettere S, Harrison L, Mcneil M, Heu R, Neiderer A (1993) Structure of electroless nickel coatings. Plat Surf Finish 80(6):60–64
Timothy CS, Andrew CT, Baumgart P, Colonia J (1998) Bump formation and growth by multiple laser pulses on Ni–P disk substrate. IEEE Trans Magn 34(4):1786–1788
Wei-Yu C, Jenq-Gong D (2004) Thermal stability of sputtered Ni–P and Ni–P–Cr coatings during cycling test and annealing treatment. Surf Coat Technol 177–178:222–226
Zou D, Yan D, Xiao L, Dong Y (2008) Characterization of nanostructured TiN coatings fabricated by reactive plasma spraying. Surf Coat Technol 202:928–934
Acknowledgments
One of the authors, Dr. Atikur Rahman would like to thank Prof.(Dr.) Rajat Gupta, DIRECTOR, NATIONAL INSTITUTE OF TECHNOLOGY SRINAGAR HAZRATBAL, KASHMIR-190006, INDIA for their financial support to this work (Order No. 189 of 2012,Dated:04-04-2012).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution License which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
About this article
Cite this article
Rahman, A., Jayaganthan, R. Effect of pH values on nanostructured Ni–P films. Appl Nanosci 5, 493–498 (2015). https://doi.org/10.1007/s13204-014-0342-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13204-014-0342-1