Abstract
This review looks at the main processes available for the production of light olefins with a focus on maximizing the production of propylene. Maximization of propylene production has become the focus of most refineries because it is in high demand and there is a supply shortage from modern steam crackers, which now produce relatively less propylene. The flexibility of the fluid catalytic cracking (FCC) to various reaction conditions makes it possible as one of the means to close the gap between supply and demand. The appropriate modification of the FCC process is accomplished by the synergistic integration of the catalyst, temperature, reaction-residence time, coke make, and hydrocarbon partial pressure. The main constraints for maximum propylene yield are based on having a suitable catalyst, suitable reactor configuration and reaction conditions.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Light olefins are important raw materials in many petrochemicals because they are building blocks for many end products, such as polyethylene and polypropylene. Recently, market analysis show that the demand for propylene is outpacing that of ethylene and the current supply cannot match the demand. A large proportion of propylene is produced by steam cracking (SC) of light naphtha and during the fluid catalytic cracking (FCC) process. Figure 1 illustrates the propylene production capacity of the main technologies and their contribution in bridging the demand supply gap [17, 51].
SC is an established technology for the production of light olefins, such as ethylene and propylene. It accounts for about 60–65 % of the world’s propylene production, with the established refinery FCC process accounting for 30 % and the remainder is produced on purpose using metathesis or propane dehydrogenation [57, 76].
With the ethylene and gasoline being the main products from SC and conventional FCC, respectively, propylene and other light olefins are obtained as byproducts from these technologies. Propylene production from steam crackers depends upon the operating rates of the steam cracker and the type of feedstock. In the past, propylene was produced from steam crackers via heavy liquid cracking and as a result, it was readily available; however, most modern steam crackers use ethane-based feed in place of heavy liquids leading to less propylene being produced as illustrated in Table 1 [18]. From Table 1, it is expected that propylene production from steam crackers will be lower than the corresponding ethylene production as a result of the shift to ethane-based feed.
As highlighted in Fig. 1 and Table 1, it can be seen that SC alone cannot satisfy the demand for propylene. Therefore, there is need of new technology to produce additional propylene to bridge the gap between supply and demand.
With on purpose propylene production technologies, such as propane dehydrogenation and metathesis being touted as possible alternatives, the cost associated with these technologies remains less competitive relative to steam crackers and FCC.
It could have been easier to fill the gap by reconfiguring the steam cracker, but the steam cracker does not provide flexibility of operation and it has high energy consumption. It is the most energy consuming process in the chemical industry and uses approximately 8 % of the total global primary energy use, excluding energy content of final products [89]. According to Ren et al. [89], the pyrolysis section of a naphtha steam cracker alone consumes about 65 % of the total process energy and contributes to about 75 % of the total energy loss.
Being an essentially non-catalytic and nonselective process SC is energy intensive and catalysts have never been widely used in the pyrolysis section in SC to optimize energy efficiency. By adopting technologies based on the reconfiguration of the FCC unit to maximize the production of propylene and light olefins, it is expected that energy savings and flexibility of operation will be obtained because [58, 89]:
-
First, FCC catalysts provide an alternative route to SC with the use of lower activation energy for C–C bonds rupture. Consequently, the temperatures for the new catalytic naphtha cracking processes are 150–250 °C lower than those for steam crackers.
-
Second, catalysts improve selectivity to desired products, such as propylene. Even if the same operating conditions as those of SC are applied for catalytic cracking, the total olefin yield would still be enhanced by at least 15 % [4].
-
Third, coke formed during the cracking process is constantly removed by catalysts that are in turn decoked through catalyst regeneration or catalyst decoking.
-
Fourth, FCC is one of the most flexible processes in a refinery and can readily adjust to changes in feed quality through modifications to catalyst and operating conditions.
The configuration of the FCC process, which involves a circulating fluidized bed with the availability of heat and mass transfer and catalysts regeneration, makes it possible for the FCC to be used for applications that go beyond the upgrading of heavy feed to gasoline.
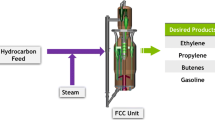
In the FCC, light olefins are produced via catalytic cracking of hydrocarbon feedstocks by contacting the feed with a catalyst usually consisting of one or more crystalline microporous molecular sieves to selectively convert the feed into an olefin containing mixture.
The propylene demand from FCC is growing at a faster rate than global FCC capacity and therefore propylene yields from FCC need to increase to keep up with demand. Figure 2 illustrates the production of additional propylene due to the advent of on-purpose FCC technology.
The objective of the present review is to evaluate the processing of hydrocarbon feedstocks to produce propylene and summarize the effects of existing FCC technology, operation variables and catalysts on product quality and quantity. These three main factors form what is called the constraint triangle for maximizing propylene production as described by Maadhah [65]. The effect of each factor is discussed and supported by experimental results in the literature.
Variables that affect propylene production
Reactor configuration
New FCC catalyst technologies are being developed to enable refiners to achieve the challenging propylene yields required to meet the growing demand for propylene from FCC. As a result, various methods and configurations have been proposed for increasing or enhancing the output of propylene product stream from the FCC unit (see Fig. 3).
FCCU design and operating modes [32]
By taking into consideration the operating conditions and yields of the FCC, the propylene yield pattern can be represented in the form of a continuum varying from operating severity to process design and these can be optimized to suit the refinery specific economics [32]. The optimum process design provides refiners with the flexibility to move up or down the optimal economic range of the propylene yield curve as shown in Fig. 3. From Fig. 3, it can be seen that higher propylene production comes at the expense of gasoline. For traditional refiners, maximizing gasoline yield is more important than the propylene yield, while for those interested in petrochemical applications, the target is operating at maximum propylene yield.
With the strong market demand for propylene and the capability to achieve elevated propylene yields via FCC technology, there is a natural desire to go for maximizing propylene yields.
Riser and downer FCC
Many FCC processes increase propylene by manipulating FCC reaction variables such as catalyst to oil (C/O) ratios, residence times and reaction temperatures [39]. The modifications can be put into two categories: Up Flow (Riser) and Down Flow (Downer) technologies. In the riser reactors, solid catalyst and hydrocarbon vapors flow upwards against gravity. This upward flow results in a catalyst flow that is significantly slower than the lighter hydrocarbons leading to back mixing of the catalyst and as a result there is an increase in residence time of the catalyst. This in turn can lead to undesirable secondary reactions leading to over cracking. The illustration of the flow pattern in the riser and downer is shown in Fig. 4 [6].
Illustration of flow in riser and downer FCC [6]
In contrast to risers, and to overcome the issues related to back mixing, the downer reactor was developed as illustrated in Fig. 4. The flow of the catalyst and the feed is in the direction of gravity and as such, back mixing is largely avoided and there is an even distribution of catalyst with an effective contact time of catalyst and feed less than that of the riser.
The FCC technology based on the downer design, and which is in commercial operations, is briefly described below.
Downer FCC technology: high severity fluid catalytic cracking (HS–FCC)
The HS–FCC process developed jointly by Saudi Aramco and its partners is operated under considerably higher reaction temperatures (550–650 °C) than conventional FCC units and the main objective is to produce more propylene and high octane number gasoline [3, 5, 24, 41, 64, 65, 82]. Under these conditions, however, thermal cracking of hydrocarbons also takes place concurrently with catalytic cracking, resulting in increased undesirable products as dry gas and coke. Short contact time (less than 0.5 s) of the feed and product hydrocarbons in the downer minimizes thermal cracking. Undesirable successive reactions, such as hydrogen transfer, which consume olefins, are suppressed. To attain the short residence time, the catalyst and the products have to be separated immediately at the reactor outlet. For this purpose, a high efficiency, short residence time product separator has been developed, and is capable of suppressing side reactions (oligomerization and hydrogenation of light olefins) and coke formation [5, 6, 24, 41, 65].
Due to the short contact time, the conversion in HS–FCC mode is expected to drop and to compensate for this, the HS-FCC process is operated at a high C/O ratio and at higher temperatures than the conventional FCC. The advantage of operation at a high C/O ratio is the enhanced contribution of catalytic cracking over thermal cracking. High C/O maintains heat balance and helps minimize thermal cracking, over cracking, and hydrogen transfer reactions. The synergetic operation of the reaction conditions, high C/O ratio and downer operation guarantee a high olefin.
Riser FCC technology
Two technologies based on Riser FCC are deep catalytic cracking (DCC) and catalytic pyrolysis process (CPP), developed by SINOPEC.
-
a.
Deep catalytic cracking (DCC)
DCC is derived from FCC and its flow scheme is similar to that of FCC consisting of a continuous reaction/regeneration system with fluidized catalyst circulation [68, 83, 92, 93, 100, 103, 105]. The main difference in hardware is a bed reactor installed after the riser. DCC uses FCC principles with specific enhancements to produce large yields of light olefins and high octane naphtha. To achieve a high olefin yield, a high reactor temperature is required. The DCC unit operates at temperatures as high as 570 °C, somewhat higher than maximum olefin FCC and residue FCC operations.
-
b.
Catalytic pyrolysis process (CPP)
CPP is further modified from DCC aiming at more ethylene production. The modification includes new catalyst formulation, varied operating conditions and some changes on engineering [100, 105].
CPP catalyst possesses the features of low hydrogen transfer reaction; high matrix activity; active component consisting of both large pore and meso-pore zeolites; modification of the meso-pore zeolite to increase the ratio of Lewis acid to Bronsted acid for enhancing free radical reaction; higher hydrothermal stability and lower attrition index. CPP operating conditions are more severe than that of DCC. In comparison with DCC, the reaction temperature is about 80 K higher, therefore, it requires higher regeneration temperature to provide the heat of reaction; and both the steam dilution and catalyst to oil ratio are double. CPP uses a riser reactor, a quenching technology, and a cross current degassing device to minimize the flue gas adsorbed and entrained by the regenerated catalyst (see Table 2).
DCC and CPP use more steam than conventional FCC (Table 3) and their operation can be termed as steam catalytic cracking (SCC). SCC is a process of cracking hydrocarbons to light olefins in mild temperatures in the presence of steam over a catalyst. SCC combines mild thermal cracking with the acid promoted cracking of a zeolite-based catalyst, and can provide very high yields of light olefins (with the possibility of varying the propylene-to-ethylene ratio) while operating at temperatures much lower than those used in the SC process [104]. The main feed for the SCC process so far has been naphtha or other light feed [5, 9, 29–31, 65, 67, 89, 104], but the amount of coke produced during cracking of naphtha, or similar feeds, is too low to produce heat by combustion to maintain the catalyst temperatures required in the reactor. Therefore, extra heat will have to be supplied into the regenerator by burning off added hydrocarbons or more coke would have to be produced by using suitable catalyst and heavier feedstocks as a solution for heat balancing the SCC.
Characteristics of some FCC technologies
Table 2 illustrates the main characteristics of the FCC-based technologies compared to those of SC.
Most of the new FCC based technologies for SCC make use of high C/O ratios to promote catalytic cracking and reduce thermal cracking. Using a high C/O ratio also guarantees that more heat is transferred from the regenerator to the reactor as the catalyst and oil will equilibrate at higher temperatures in the reactor.
Apart from the DCC, all the other techniques have shorter residence times in the reactor than the normal FCC and again, this is based on the triple constraints in the triangle in Fig. 3. For the CPP and HS–FCC, which operate at higher temperatures, the advantage of shorter residence time is to prevent over cracking, which can lead to the production of more ethylene. While for the DCC, a longer contact time is required to guarantee the cracking of the reactants.
The product distribution from these technologies is summarized in Fig. 5 from which it can be seen that all the FCC modified processes produce more propylene than the main FCC process. Also, apart from the HS–FCC process, more coke is produced showing that these processes are capable of achieving the heat balance needed during steady-state operation. Another observation is the fact that all the FCC-based processes produce less gasoline, especially the CPP process. This shows that if the FCC-based processes are fully integrated into the refinery system, there is a possibility of having a shortage of gasoline in the market. This therefore requires that a balance be made between maximum propylene yield and guaranteeing gasoline supply. One way of guaranteeing gasoline production is to look at using crude oil as feed so that refinery capacity should not be a restricting factor for the new processes.
While propylene generation from an FCCU certainly varies with feedstock, it is primarily a function of catalyst type, reactor temperature, partial pressure, C/O ratio and total pressure.
Catalyst composition
Catalyst structure
One of the factors that affects the design and operation of an FCC unit is the type of catalyst to be employed in the process. Most FCC catalysts consist of an active component (zeolite), a matrix such as amorphous silica-alumina (which also provides catalytic sites and larger pores), a binder (such as betonite clay) and filler, which provides physical strength of the catalyst [7, 42, 50, 68, 84, 95, 102, 104]. Ultra-stabilized zeolite Y (USY) is used as the main active zeolite in today’s conventional FCC catalyst, which consists of different phases as shown in the schematic representation in Fig. 6 [84]. It is composed of spherical particles, suitable for application in a fluidized circulating reactor, in which the zeolite crystals are dispersed in an active matrix of alumina or silica-alumina together with clay particles. The spherical particles contain large voids and pores necessary for allowing the mass transport of the heavy feedstock.
Schematic representation of FCC catalyst [84]
The matrix of an FCC catalyst serves both physical and catalytic functions [95]. Physical functions include providing particle integrity and attrition resistance, acting as a heat transfer medium, and providing a porous structure to allow diffusion of hydrocarbons into and out of the catalyst microspheres [7, 42, 47, 95, 102, 104]. The matrix can also affect catalyst selectivity, product quality and resistance to poisons.
The matrix tends to exert its strongest influence on overall catalytic properties for those reactions, which directly involve large molecules.
FCC catalysts also have a hierarchical pore architecture spanning from the macro- to meso- to microporosity with an illustration shown in Fig. 7 [79]. Each of these classes of pores has a defined role in the entire catalytic process. According to this scheme, the transformation of heavy molecules to valuable products (gas-oil and gasoline) occurs in the meso- and micropores [10, 56, 59, 62, 63, 66, 73, 78, 79, 87, 88, 90, 97].
Schematic representation of the hierarchical pore structure in catalyst [79]
Although the FCC unit was developed purposely to help in the conversion of low value feed into more gasoline, the unit and the process have undergone several modifications, some of which are aimed at tackling the increasing demand for some of it byproducts, such as propylene. Propylene used to be a byproduct from the FCC unit, but recent market trends have made it possible for the redesign of the FCC unit, subsequently upgrading propylene from a byproduct to a co-product. This has also meant a redesign of the catalyst that will enable the production of more propylene.
For the modern conventional FCC process, the desired catalyst properties are:
-
Good stability to high temperature and to steam [5, 29, 30, 31, 43–45, 52, 61, 65, 67, 69, 99, 104, 106]. The catalysts must have the thermal stability to maintain particle and catalytic integrity under severe regenerator conditions.
-
High activity to carry out conversion of the feed before any significant amount of thermal cracking sets in. Thermal cracking leads to undesirable products such as methane, ethane and some propane. On the other hand, catalytic cracking produces relatively fewer C 1 and C 2 fragments and a larger number of olefins are produced.
-
Large pore sizes to crack larger molecules so that they can get into smaller pores.
-
Good resistance to attrition to maintain particle morphology under the severe impact and erosion forces that exist in the FCC unit.
-
Low coke production so the catalyst can remain active for a longer period.
One catalyst that has been incorporated into the FCC catalyst formulation for the production of light olefins is ZSM-5. Table 3 shows the chronology of catalyst and additive development for light olefins enhancement in the FCC.
ZSM-5 additive for olefin production
-
a.
Effect of ZSM-5 amount
As the unit operating severity is limited by mechanical constraints and the choice of feed is characteristic of the source of crude oil, the selection of the optimum catalyst system is critical in maximizing both the desired yield and unit profitability [8]. This requires a thorough understanding of the unit constraints and limitations, as well as the feed quality and yield objectives. The optimum catalyst system will maximize both propylene selectivity and gasoline olefinicity, while minimizing hydrogen transfer, isomerization, oligomerization and aromatization reactions. For the purpose of producing more propylene and olefins, more ZSM-5 is being used as the main active component of the catalyst in the FCC unit [2, 11, 19, 33, 108].
Bulatov and Jirnov [20] analyzed feed conversion over varying concentrations of a component additive containing ZSM-5. The additive level was varied from 0 to 40 % over a C/O ratio of about 28, a riser outlet temperature of 566 °C, a riser partial pressure of 0.0793 MPa, and a contact time of 1.5 s. From the analysis, it was observed that increasing the amount of ZSM-5 to very high levels had only a marginal effect on the production of propylene as shown in Fig. 8.
Effect of ZSM-5 loading on propylene yield [20]
Propylene yield tends to plateau with about 10 % ZSM-5 crystal concentration in the catalyst inventory. This is explained by the fact that the diminishing effectiveness of ZSM-5 at higher concentrations occurs primarily due to the depletion of the gasoline olefin precursors. ZSM-5 generates propylene by selectively cracking olefins in the gasoline boiling range. As the concentration of ZSM-5 additive in the catalyst inventory increases, the incremental yield of propylene produced per percentage of additive decreases.
Crystal size (diffusion path length) and the Si/Al ratio (catalyst acidity) of ZSM-5 will also affect the yield of propylene and to suppress large transition state high reaction order, undesirable hydrogen transfer and aromatization reactions, the acid sites need to be quite far apart and the crystal size needs to be small.
-
b.
Effect of crystal size
The main factor allowing molecular sieving, and consequently, the shape selectivity is generally considered to be exclusively a steric effect, i.e., only molecules having a critical kinetic diameter lower than the channel diameter are allowed to enter the pores and to react on an active site, or to exit them and to be recovered as a product of the reaction [12]. Alternatively, transition state shape selectivity effects limit the formation of bulky transition state intermediates inside the pores and avoid the formation of some unwanted reaction products. In a heterogeneous catalytic reaction involving large molecules, diffusion of these large molecules to the catalytic active internal sites of the zeolites will become a rate limiting process. More secondary products and faster deactivation were observed due to longer intra-crystalline diffusion path lengths [23, 48, 75, 77].
One method of overcoming these diffusional limitations is to reduce the particle size of zeolites and shorten the diffusional paths [37]. In ZSM-5 there exists a remarkable molecular sieving effect for light hydrocarbons and this has been widely used as shape selective catalysts in various hydrocarbon processes; however, because the crystal sizes of ZSM-5 are usually much larger than the sizes of the micropores, the rate limiting step of the reaction tends to be the diffusion of the reactant/product molecules within the micropores [40, 54, 101]. Moreover, carbon solid (coke) readily forms near the external surface of the crystal under diffusion controlled conditions, thereby, rapidly plugging the pores, leading to a short catalyst lifetime. To achieve low diffusion resistance, nano-sized zeolites are effective because the diffusion length for reactant/product hydrocarbons, which depends on the zeolite crystal size, is reduced [55, 74, 98]. High propylene selectivity from cracking of naphtha is favored over larger 10-membered ring zeolites having a pore index between 26 and 30. The pore size index is defined as the product of the two principal dimensions, or diameters, of the pore and is in units of square Angstroms (Å2).
Konno et al. [54], studied the effect of zeolite crystal size on the catalytic stability using ZSM-5 zeolites (Si/Al = 150) in n-hexane cracking. Their results are summarized in Fig. 9. From their results, it can be seen that the initial conversion of n-hexane was almost the same (approximately 94 %) regardless of the crystal sizes, and the highest ethylene + propylene yield obtained was 53.5 C-mol % with a propylene/ethylene ratio of 1.57 at 94.1 % conversion over MFI(S)150. Subsequently, the conversion gradually decreased with time onstream over MFI(L)150, decreasing to 48 % after 50 h. In contrast, MFI(S)150 and MFI(M)150 maintained high conversions at 82 and 81 %, respectively, after 50 h, and were hardly changed from the start of reaction. Moreover, the stable activity of the nano-zeolites (MFI(S)150 and MFI(M)150) gave stable product selectivities compared with the macro-zeolite (MFI(L)150).
n-Hexane cracking over ZSM-5 zeolite (Si/Al = 150) with different crystal sizes [54]
-
c.
Effect of Si/Al
ZSM-5 zeolite has a unique three-dimensional structure, with very small pores compared to the Y-zeolite in a normal FCCU catalyst. This makes ZSM-5 zeolite “shape selective” for cracking the long chain (C 6–C 10) olefin molecules in FCCU gasoline (it also cracks the equivalent paraffin molecules but at a much slower rate). The products of these cracking reactions are predominantly propylene and butylene, with a small amount of isobutane [86]. Changing the Si/Al ratio in ZSM-5 translates to altering the ratio of cracking/isomerization rates.
Catalytic active sites also exist on the external surface and at the pore mouth of zeolite crystals. For shape selective reactions, these sites are considered to be responsible for unwanted nonselective catalysis [81]. Most hydrogen transfer reactions in ZSM-5 occur on the surface of the catalysts and are more pronounced at low Si/Al ratios when acidity is high [13, 35]. These hydrogen transfer reactions lead to the production of more dry gas, such as methane and ethane, leading to a drop in the selectivity of light olefins. It is thought that a smaller crystal size in combination with high Si/Al ratio gives higher light olefins yields due to lower residence time of primary products in the pores of the catalyst in contact with the acid sites [109].
The stability of the catalyst is also affected by the Si/Al ration especially in relation to coke formation. It has been proven that the higher the Si/Al (lower acidity), the smaller the amount of coke form, with the knock-on effect being the extended catalyst lifetime. This is directly linked to the fact that coke deposition is dependent on hydrogen transfer reactions, which in turn is dependent on the catalyst acidity. If the catalyst acidity is suppressed, then the rate of coke deposition is reduced.
-
d.
Hydrothermal stability of ZSM-5
The main cause of ZSM-5 deactivation is de-alumination due to the presence of steam at high temperatures, which leads to a partial destruction of its framework structure [5, 29, 30, 31, 43–45, 52, 61, 65, 67, 69, 99, 104, 106]. To overcome of this problem, phosphorus impregnation has been used to stabilize the ZSM-5 structure. Several studies have reported changes on the hydrothermal stability after impregnation with phosphorus not only for ZSM-5 zeolites but also for FAU and MOR zeolites [5, 29, 30, 31, 43–45, 52, 61, 65, 67, 69, 99, 104, 106]. Even so, before the steaming treatment, the impregnation with phosphorus was said to produce several counterproductive effects [22]:
-
Reversible decrease in activity due to the interaction of P species with the protonic sites;
-
External surface blockage;
-
Decrease in the microporous volume; and even
-
De-alumination.
Despite these setbacks, the phosphorus impregnated samples seemed to retain their acidity and activity during the steam treatment to a higher level than the untreated zeolite. This means that the phosphorus species formed in the treatment reinforce the zeolite structure and prevent de-alumination. The optimal phosphorus content (highest activity) obtained depends essentially on the zeolite (Si/Al) ratio and on the model reaction used [16, 21]. For example, Blasco et al. found a maximum in the n-decane cracking activity for P/Al molar ratios of 0.5–0.7 [16].
If normal FCC catalysts have to be adopted for processes using SCC, then the catalysts would have to be highly hydrothermal and this should be achieved without necessarily compromising the yield to ethylene and propylene [29, 30].
-
e.
Coke formation
FCC processes are usually accompanied by the production of coke and all heterogeneous acid catalyzed reactions of organic compounds result in deactivation due to coking. Coke can be defined as compounds with hydrocarbon ratio of 0.3–1.0 and it is made up of many components, which are nonvolatile, with low boiling points and low hydrogen content.
Coke is generally formed as a result of a sequence of elementary reactions, which are affected by the type of reaction, feed composition, type of catalyst and reaction-reactor environment. In addition, a range of factors will affect the composition of coke, including the nature of the reactants, time on-stream, temperature, acid site concentration and the location of the coke deposit [15, 22, 34, 38]. Therefore, coke will have a broad range of compositions, determined by these different factors.
There are five main types of coke identified in catalytic cracking [22, 34, 38].
-
Catalytic coke—from condensation and dehydrogenation.
-
C/O coke—hydrocarbons entrained in the small pores and not removed by the stripper.
-
Thermal coke—formed by a free radical mechanism, it is important at high reaction temperatures and also yields hydrogen. It is less important than catalytic coke due to the low extent of thermal cracking at typical FCC conditions.
-
Additive coke (or Conradson coke)—from heavy molecules already present in the feed. Its amount correlates directly with the Conradson carbon residue (residue remaining after the fuel has been pyrolyzed by raising the temperature to 800 °C).
-
Contaminant coke—from dehydrogenation catalyzed by Ni, Fe and V.
Coke formation is highly complex and probably involves precursors of various types, as well as many chain reactions and rearrangements inside the channels and cavities and/or on the external surface of the catalyst. Therefore, it is a very important consideration when acidic zeolite catalysts are used. When deciding which catalyst and which process to use, it is essential to understand fully the mechanisms that control coking and the effect it has on catalytic properties, such as activity and selectivity. In most industrial processes, catalyst deactivation is as important a consideration as controlling the activity and selectivity, because it is extremely costly. It is therefore a fundamental economic objective to limit deactivation by coking and also to regenerate catalysts. The problems relating to facilitating the stability of catalysts and optimizing their regeneration need to be investigated in both industrial and academic laboratories to find both technical and conceptual solutions.
It is known that in zeolites, pore size, pore structure and acidity affect coke deposition [44]. The ZSM-5 zeolite has a lower tendency to form coke, compared to the Y zeolite, due to its narrow pores that limit the formation of bulky coke intermediates.
After considering the reactor system and catalyst formulation to go with it, the next thing that will ultimately affect conversion and product selectivity is the reaction variables.
Reaction variables
Effect of contact time or catalyst circulation rate
Residence time in the reactor varies according to the reactor configuration, reaction temperature, C/O ratio and the intended product. Taking the HS–FCC configuration for example, short contact time is required to prevent a secondary reaction involving hydrogen transfer reactions from occurring [6]. In terms of residence time distribution, the conventional FCC has a higher residence time distribution than the HS–FCC process. This is because the HS–FCC process uses a higher C/O ratio, higher temperature and it is aimed at maximizing propylene production to prevent thermal cracking and hydrogen transfer reactions from taking place as illustrated in Fig. 10.
Outside the optimum residence time window, there is the possibility of producing less propylene than anticipated because there is low conversion at lower residence time, and over cracking at higher residence time.
Meng et al. [70] studied the effect of contact time on product distribution at 650 °C using VGO and CEP-1 catalyst and their results are shown in Table 4.
The feed conversion was about 98.5 % and remains relatively constant with residence time. The yields of light olefins first went up until a residence time of about 2.0 s, where they remained relatively constant.
A longer residence time indicates that there was more time for catalytic pyrolysis of hydrocarbons, and therefore the pyrolysis extent was more thorough. At these experimental conditions, the reaction rates of heavy oil catalytic pyrolysis were very fast. The LPG was susceptible to secondary cracking reactions to produce dry gas, and accordingly, the yield of LPG decreased as residence time increased. At the same time, the yields of dry gas, gasoline and diesel oil increased.
Effect of temperature
A rise in temperature will increase the extent of catalytic cracking. In a commercial unit, the reaction temperature is raised by raising the catalyst circulation. By using a higher C/O ratio, the reaction rate of the catalytic cracking is improved and the propylene yield increases. To control the hydrogen transfer reaction, it is better to use a short contact time since it is a secondary reaction. Reactions with only short contact times will also control overcracking [46].
HS–FCC units operating at maximum propylene production use short contact time along with high reaction temperature and higher C/O ratio. This is to accelerate catalytic cracking, limit thermal cracking and control the hydrogen transfer [6, 20].
Meng et al. [70] studied the effect of temperature on feed conversion, selectivity of total light olefins and product distribution in the reaction temperature range of 600–716 °C using a CEP-1 catalyst. The results from their study are summarized in Fig. 11.
Effect of reaction temperature on product distribution [70]
From the results in Fig. 11, as the temperature goes up, the yield of dry gas increases, while that of propylene and butylene decreases. Secondary reactions by propylene and butylene increase as a consequence of further pyrolysis, due to temperature increase.
According to the experimental data, ethylene yield increases with the increase of reaction temperature, while the yields of propylene and butylene pass through maxima. Propylene and butylene are mainly generated through cracking mechanism via the carbonium ion and because they are intermediate products, they can undergo secondary reactions such as cracking and hydrogen transfer, especially at high temperatures. So from their results, to achieve a high propylene yield, the optimum temperature range is 620–660 °C and the propylene yield is much higher than that of ethylene.
Effect of C/O ratio
The amount of catalyst that contacts the feed will vary depending on the temperature of the regenerated catalyst and the severity of the FCC process. A high C/O ratio will operate to maximize conversion, which tends to favor light olefin production [71, 80, 94].
Although it has been well established within the art of FCC that increasing C/O ratios will increase conversion, C/O ratios cannot be easily increased since this ratio is not an independent variable in standard FCC units. Rather the C/O ratio is dependent on the heat balance limitations of the unit. Consequently, only relatively low C/O ratios of 4–10 are typically observed for conventional FCC. Such a means of increasing C/O ratios, however, was not expected to maintain high catalyst activities due to the coke deactivation of the catalyst. Reducing the C/O ratio results in an increased light olefin yield and a decreased dry gas yield.
An example of the effect of C/O ratio is illustrated in the study carried out by Meng [70] and shown in Table 5. As the C/O ratio goes up, the feed conversion and the yields of dry gas and coke increase, that of gasoline and diesel oil decrease, while that of LPG shows a maximum at about 17. From the data, there is little variation in the yields of light olefins with increasing C/O ratio. It shows a slight increase in ethylene yield and a slight decrease in the butylene yield. Also, yields of propylene and total light olefins pass through maxima and the selectivity to light olefins reaches its highest value of 49.42 % at around a C/O of 13.
A large C/O ratio means that reaction will occur at a higher temperature as the catalyst and feed will equilibrate at high temperatures. This means much energy can be transferred in the reaction process, which can accelerate thermal cracking reactions. To a certain extent, a high C/O ratio means a thorough pyrolysis as this can promote secondary reactions of light olefins and may affect production cost. Therefore, the value of the C/O ratio cannot be too high and should be optimized based on the FCC technology being used.
Effect of feed quality
Feedstocks that are high in aromatics have low hydrogen content and therefore are resistant to conversion at typical FCC residence times. The production of propylene requires a disproportionate share of the hydrogen and co-products, including propane, and dry gas requires an even greater share of hydrogen. Therefore, the amount of hydrogen available from the feedstock can limit the potential to produce propylene. Subsequently, propylene production is highly dependent on feed properties. Conradson carbon is another important factor as much of the Conradson carbon ends up as coke, thereby further reducing the potential propylene production.
Meng et al. [70] were able to show the effect of feedstock quality on product distribution by investigating four types of feeds using a CEP-1 catalyst at a reaction temperature of 660 °C, residence time of 2.2 s, C/O weight ratio of 15.5 and steam-to-oil weight ratio of 0.75. Their results are summarized in Table 5:
From the results in Table 6, they found that the feed conversion of the four kinds of heavy oils remained very high, above 98 %. They also observed that as the hydrocarbon mol ratio was increasing with a corresponding decrease in aromatic carbon content, the yields of dry gas, diesel oil was decreasing, but the coke yield showed an increasing trend. The yields of LPG and light olefins together with the selectivity of overall light olefins show an increasing trend.
In general, more propylene can potentially be derived from feed sources that are hydrogen rich and low in contaminants because of the relative ease of conversion [8, 70]. Feed sources rich in aromatic components produce fewer olefin precursors in the gasoline boiling range, resulting in potentially less propylene yield.
Effect of hydrogen transfer index
The hydrogen transfer index is defined as the paraffin/olefin ratio of C 3, linear C 4 and branched C 4 species. The relative activity of FCC catalysts for generating secondary reactions can be estimated using the hydrogen transfer index (HTI) for catalysts tested under constant conditions with the same feed [1, 8, 27, 36, 60, 91, 107]. Catalysts with lower HTIs generate fewer secondary reactions, preserving a greater quantity of the gasoline boiling range olefins, which can be subsequently cracked to lighter olefins. Suppressing hydrogen transfer by maximizing the availability of olefin precursors is the key to maximizing propylene.
Hydrogen transfer reactions involve the formation of bulky bimolecular reaction intermediates, and are mainly controlled by steric constraints, due to the space available inside the micropores of the zeolites [28]. They can also occur on the outer surface of the zeolite particles. The smaller the pore size of the zeolite, the greater the extent of the suppression of the hydrogen transfer reactions of the alkenes, which means that the HTI decreases with the pore size of the zeolite.
Zhu [109] studied the effect of pore size on hydrogen transfer activity and from their results, they showed that hydrogen transfer activity decreases as the pore size of the zeolite decreases. For the zeolite studied, they showed that the HTI decreased in the following order: Y > Beta > MCM-22 > ZSM-5.
Catalyst properties can be modified by several techniques to suppress hydrogen transfer reactions. Controlling the zeolite acid site density and optimum dispersion of these acid sites is crucial in minimizing hydrogen transfer [14, 72, 85]. Reducing the number of acid sites may be accomplished by minimizing the zeolite unit cell size (UCS) [85]. The zeolite UCS may be reduced by several techniques; as the alumina ions are removed, the intrinsic activity of the zeolite decreases.
Hydrogen transfer activity may also be mitigated by producing a zeolite with a highly accessible pore structure, which enhances diffusion. A catalyst designed with high accessibility allows the olefin produced from primary cracking to rapidly diffuse from the catalyst particle. Since hydrogen transfer is a bimolecular reaction requiring the reactants to be in close proximity to a pair of acid sites, reducing the residence time of olefins within the catalyst particle reduces the hydrogen transfer rate. For small pore zeolites, the residence time can be reduced by using nanocrystals.
Effect of hydrocarbon partial pressures
It is generally expected that a rise in hydrocarbon partial pressure will increase the rate of all bimolecular reactions, including hydrogen transfer, relative to cracking, which is unimolecular [49]. An increase in the rate of hydrogen transfer will result in a reduction of olefins in both gasoline and LPG, and an increase in gasoline range aromatics and paraffins. The change in the rate of hydrogen transfer could also affect the gasoline sulfur concentration as well as the effectiveness of gasoline sulfur reduction catalysts and additives. Moreover, the effectiveness of ZSM-5 additives, which are used to produce light olefins, especially propylene, could be affected by the hydrocarbon partial pressure. As ZSM-5 works by cracking gasoline range olefin molecules, changing the rate of hydrogen transfer could have a profound impact on the propylene yield.
Hu [49] studied the effect of hydrocarbon partial pressures on propylene yield by using a Davison circulating riser (DCR) and their results are summarized in Table 7.
From their results in Table 7, they found that raising the hydrocarbon partial pressure increased the amount of dry gas and coke at the expense of gasoline. They attributed the higher coke yield to a higher rate of oligomerization, which is a bimolecular reaction and favored at high pressure. They also found that increasing the hydrocarbon partial pressure substantially lowered the C 3 and C 4 olefinicities leading to a decrease in the yield of propylene and butylene. These yield shifts suggest that the rate of hydrogen transfer increases with hydrocarbon partial pressure, as would be expected for a bimolecular reaction [26, 49].
These observations are consistent with the notion that hydrogen transfer reactions, being bimolecular in nature, increase with rising hydrocarbon partial pressure. The hydrogen transfer increased with the hydrocarbon partial pressure. They also demonstrated that the effectiveness of ZSM-5 additives was lessened at high hydrocarbon partial pressure due to the depletion of gasoline range olefins via hydrogen transfer reactions.
Conclusions
The main constraints for maximum propylene yield are based on having a suitable catalyst, suitable reactor configuration and reaction conditions. The FCC process is modified by the synergistic integration of the catalyst, temperature, reaction-residence time, coke make, and hydrocarbon partial pressure. Achieving maximum propylene and conversion from a wide range of feed qualities offers considerable challenges to the catalyst design. The impact of feed composition and process variables on the yields and heat balance is significant and therefore requires a good understanding of the chemistry to help with designing the right FCC catalyst for a unit. To guarantee high propylene yield, a good catalyst must have low hydrogen transfer, high accessibility and prevailing matrix technology to complement the right reaction system and reaction conditions.
Global propylene demand trends remain strong, and with the change toward lighter feedstocks in modern steam crackers there will be a growing dependence on the FCC to balance the supply side of the propylene equation. Some of the technologies described in this review are those that have been taken to the commercial stage such as DCC and HS–FCC, and are by no means an exhaustive list because there is a rising interest and ongoing research in applying special reactors and catalysts to control the yield of olefins and improve energy efficiency. The future development of catalytic olefin technologies would be strongly affected by the market forces and feedstock cost competition.
References
Abbot J, Wojciechowski BW (1987) Hydrogen transfer reactions in the catalytic cracking of paraffins. J Catal 107:451–462. doi:10.1016/0021-9517(87)90309-5
Abul-Hamayel MA, Aitani AM, Saeed MR (2005) Enhancement of propylene production in a Downer FCC operation using a ZSM-5 additive. Chem Eng Technol 28:923–929. doi:10.1002/ceat.200407133
Abul-Hamayel MA, Siddiqui MA-B, Ino T, Aitani AM (2002) Experimental determination of high-severity fluidized catalytic cracking (HS-FCC) deactivation constant. Appl Catal A Gen 237:71–80. doi:10.1016/S0926-860X(02)00294-6
Aguado J, Serrano DP, Escola JM, Garagorri E (2002) Catalytic conversion of low-density polyethylene using a continuous screw kiln reactor. Catal Today 75:257–262. doi:10.1016/S0920-5861(02)00077-9
Aitani A, Yoshikawa T, Ino T (2000) Maximization of FCC light olefins by high severity operation and ZSM-5 addition. Catal Today 60:111–117. doi:10.1016/S0920-5861(00)00322-9
Al-Ghrami M, Lambert N, Vogel SR (2011) Go for propylene in the Middle East case studies. Paper presented at the Middle East Downstream Week, Abu Dhabi
Al-Khattaf S (2002) The Influence of alumina on the performance of FCC catalysts during hydrotreated VGO catalytic cracking. Energy Fuels 17:62–68. doi:10.1021/ef020066a
Amano T, Wilcox J, Pouwels C, Matsuura T (2012) Process and catalysis factors to maximise propylene output. Catal Addit 139:1–11
Andersen J (2005) Technologies for filling the propylene gap. 25–29 Sept 2005
Andreu P (1993) Development of catalysts for the fluid catalytic cracking process: an example of CYTED-D program. Catal Lett 22:135–146. doi:10.1007/BF00811774
Arandes JM, Torre I, Azkoiti MJ, Ereña J, Olazar M, Bilbao J (2009) HZSM-5 zeolite as catalyst additive for residue cracking under FCC conditions. Energy Fuels 23:4215–4223. doi:10.1021/ef9002632
Armaroli T et al (2006) Effects of crystal size and Si/Al ratio on the surface properties of H-ZSM-5 zeolites. Appl Catal A Gen 306:78–84. doi:10.1016/j.apcata.2006.03.030
Bari Siddiqui MA, Aitani AM, Saeed MR, Al-Khattaf S (2010) Enhancing the production of light olefins by catalytic cracking of FCC naphtha over mesoporous ZSM-5 catalyst. Topics Catal 53:1387–1393. doi:10.1007/s11244-010-9598-1
Bekkum HV, Flanigen EM, Jansen JC (eds) (1991) Introduction to zeolite science and practice, vol 58. Elsevier. doi:10.1016/0144-2449(92)90130-H
Bibby DM, Howe RF, McLellan GD (1992) Coke formation in high-silica zeolites. Appl Catal A Gen 93:1–34. doi:10.1016/0926-860X(92)80291-J
Blasco T, Corma A, Martínez-Triguero J (2006) Hydrothermal stabilization of ZSM-5 catalytic-cracking additives by phosphorus addition. J Catal 237:267–277. doi:10.1016/j.jcat.2005.11.011
Brookes T (2012) New technology developments in the petrochemical industry-refinery integration with petrochemicals to achieve higher value uplift. In: Egypt petrochemicals conference, Cairo-Egypt
Brooks R (2013) Modeling the North American market for natural gas liquids. In: 32nd US Association of Energy and Economics (USAEE) Conference, Anchorage, 28–31 July, 2013
Buchanan JS (2000) The chemistry of olefins production by ZSM-5 addition to catalytic cracking units. Catal Today 55:207–212. doi:10.1016/S0920-5861(99)00248-5
Bulatov RM, Jirnov BS (2009) FCC process of heavy feed stock with improved yield of light olefins Oil and Gas Business. http://wwwogbusru/eng/authors/Bulatov/Bulatov_1pdf
Caeiro G, Magnoux P, Lopes JM, Ribeiro FR, Menezes SMC, Costa AF, Cerqueira HS (2006) Stabilization effect of phosphorus on steamed H-MFI zeolites. Appl Catal A Gen 314:160–171. doi:10.1016/j.apcata.2006.08.016
Cerqueira HS, Caeiro G, Costa L, Ramôa Ribeiro F (2008) Deactivation of FCC catalysts. J Mol Catal A: Chem 292:1–13. doi:10.1016/j.molcata.2008.06.014
Chen D, Moljord K, Fuglerud T, Holmen A (1999) The effect of crystal size of SAPO-34 on the selectivity and deactivation of the MTO reaction. Microporous Mesoporous Mater 29:191–203. doi:10.1016/S1387-1811(98)00331-X
Cheng Y, Wu C, Zhu J, Wei F, Jin Y (2008) Downer reactor: from fundamental study to industrial application. Powder Technol 183:364–384. doi:10.1016/j.powtec.2008.01.022
World Light Olefins Analysis (2010) Chemical Market Associates, Inc
Corma A, Bermúdez O, Martínez C, Ortega FJ (2002) Dilution effect of the feed on yield of olefins during catalytic cracking of vacuum gas oil. Appl Catal A Gen 230:111–125. doi:10.1016/S0926-860X(01)01000-6
Corma A, Faraldos M, Mifsud A (1989) Influence of the level of dealumination on the selective adsorption of olefins and paraffins and its implication on hydrogen transfer reactions during catalytic cracking on USY zeolites. Appl Catal 47:125–133. doi:10.1016/S0166-9834(00)83268-6
Corma A, Martínez-Triguero JN, Valencia S, Benazzi E, Lacombe S (2002) IM-5: a highly thermal and hydrothermal shape-selective cracking zeolite. J Catal 206:125–133. doi:10.1006/jcat.2001.3469
Corma A, Mengual J, Miguel PJ (2012) Stabilization of ZSM-5 zeolite catalysts for steam catalytic cracking of naphtha for production of propene and ethene. Appl Catal A Gen 421–422:121–134
Corma A, Mengual J, Miguel PJ (2012) Steam catalytic cracking of naphtha over ZSM-5 zeolite for production of propene and ethene: micro and macroscopic implications of the presence of steam. Appl Catal A Gen 417–418:220–235. doi:10.1016/j.apcata.2011.12.044
Corma A, Mengual J, Miguel PJ (2013) IM-5 zeolite for steam catalytic cracking of naphtha to produce propene and ethene. An alternative to ZSM-5 zeolite. Appl Catal A 106–115:460–461
Couch KA, Glavin JP, Wegerer DA, Qafisheh JA (2007) FCC propylene production. Catal Addit Fluid Catal Crack Propylene Maximisation Q3:33–43
Degnan TF, Chitnis GK, Schipper PH (2000) History of ZSM-5 fluid catalytic cracking additive development at Mobil. Microporous Mesoporous Mater 35–36:245–252. doi:10.1016/S1387-1811(99)00225-5
den Hollander MA, Makkee M, Moulijn JA (1998) Coke formation in fluid catalytic cracking studied with the microriser. Catal Today 46:27–35. doi:10.1016/S0920-5861(98)00348-4
den Hollander MA, Wissink M, Makkee M, Moulijn JA (2002) Gasoline conversion: reactivity towards cracking with equilibrated FCC and ZSM-5 catalysts. Appl Catal A Gen 223:85–102. doi:10.1016/S0926-860X(01)00745-1
Des Rochettes BM, Marcilly C, Gueguen C, Bousquet J (1990) Kinetic study of hydrogen transfer of olefins under catalytic cracking conditions. Appl Catal 58:35–52. doi:10.1016/S0166-9834(00)82277-0
Ding L, Zheng Y, Hong Y, Ring Z (2007) Effect of particle size on the hydrothermal stability of zeolite beta. Microporous Mesoporous Mater 101:432–439. doi:10.1016/j.micromeso.2006.12.008
Dupain X, Makkee M, Moulijn JA (2006) Optimal conditions in fluid catalytic cracking: a mechanistic approach. Appl Catal A Gen 297:198–219. doi:10.1016/j.apcata.2005.09.009
Farshi A, Shaiyegh F, Burogerdi SH, Dehgan A (2011) FCC process role in propylene demands. Petrol Sci Technol 29:875–885. doi:10.1080/10916460903451985
Firoozi M, Baghalha M, Asadi M (2009) The effect of micro and nano particle sizes of H-ZSM-5 on the selectivity of MTP reaction. Catal Comm 10:1582–1585. doi:10.1016/j.catcom.2009.04.021
Fujiyama Y, Al-Tayyar MH, Dean CF, Aitani A, Redhwi HH (2007) Chapter 1 Development of high-severity FCC process: an overview. In: Ocelli ML (ed) Studies in surface science and catalysis, vol 166. Elsevier, pp 1–12. doi:10.1016/S0167-2991(07)80184-4
Gamero M P, Maldonado M C, Moreno M JC, Guzman M O, Mojica M E, Gonzalez S R (1997) Stability of an FCC catalyst matrix for processing gas oil with resid. In: Bartholomew CH, Fuentes GA (eds) Studies in surface science and catalysis, vol 111. Elsevier, pp 375–382. doi:10.1016/S0167-2991(97)80177-2
Gao X, Tang Z, Ji D, Zhang H (2009) Modification of ZSM-5 zeolite for maximizing propylene in fluid catalytic cracking reaction. Catal Comm 10:1787–1790
Guisnet M, Magnoux P, Martin D (1997) Roles of acidity and pore structure in the deactivation of zeolites by carbonaceous deposits. In: Bartholomew CH, Fuentes GA (eds) Studies in surface science and catalysis, vol 111. Elsevier, pp 1–19. doi:10.1016/S0167-2991(97)80138-3
Guisnet M, Ribeiro FR (2011) Catalytic Science Series. Deactivation and regeneration of zeolite catalysts, vol 9. Imperial College Press, London
Hamada R, Watabe M (2008) More propylene in FCC units paper presented at the annual Saudi–Japan symposium. Catalysts in Petroleum Refining Petrochemicals, Dhahran
Hargreaves JSJ, Munnoch AL (2013) A survey of the influence of binders in zeolite catalysis. Catal Sci Technol 3:1165–1171
Hoang TQ, Zhu X, Lobban LL, Resasco DE, Mallinson RG (2010) Effects of HZSM-5 crystallite size on stability and alkyl-aromatics product distribution from conversion of propanal. Catal Comm 11:977–981. doi:10.1016/j.catcom.2010.04.014
Hu R, Weatherbee G, Ma H, Roberie T, Cheng W-C (2008) Effect of hydrocarbon partial pressure on FCC propylene. Catal Addit Fluid Catal Crack Propylene Maximisation 139:1–10
Humphries A, Wilcox JR (1989) Zeolite components and matrix composition determine FCC catalyst performance. Oil Gas J USA 87(6):45–50
Hyde B (2012) Light olefins market review. In: Foro Pemex Petroquimica Mexico, 2012
Jiang G, Zhang L, Zhao Z, Zhou X, Duan A, Xu C, Gao J (2008) Highly effective P-modified HZSM-5 catalyst for the cracking of C4 alkanes to produce light olefins. Appl Catal A Gen 340:176–182. doi:10.1016/j.apcata.2008.02.011
Knight J, Mehlberg R (2011) Refining developments: maximise propylene from your FCC Unit. Gulf Publishing Company, USA
Konno H, Okamura T, Kawahara T, Nakasaka Y, Tago T, Masuda T (2012) Kinetics of n-hexane cracking over ZSM-5 zeolites—effect of crystal size on effectiveness factor and catalyst lifetime. Chem Eng J 207–208:490–496. doi:10.1016/j.cej.2012.06.157
Konno H, Okamura T, Nakasaka Y, Tago T, Masuda T (2012) Effects of crystal size and Si/Al ratio of MFI-type zeolite catalyst on n-hexane cracking for light olefin synthesis. J Jpn Petrol Inst 55:267–274
Kuehler CW, Jonker R, Imhof P, Yanik SJ, O’Connor P (2001) Catalyst assembly technology in FCC. Part II: the influence of fresh and contaminant-affected catalyst structure on FCC performance. In: GehrkOccellie ML, Connor PO (eds) Studies in surface science and catalysis, vol 134. Elsevier, pp 311–332. doi:10.1016/S0167-2991(01)82330-2
Lawler D, Letzsch W, Dhaidan F (2005) Advances in fccu technology for the production of olefins. 25–29 Sept 2005
Li X, Li C, Zhang J, Yang C, Shan H (2007) Effects of temperature and catalyst to oil weight ratio on the catalytic conversion of heavy oil to propylene using ZSM-5 and USY Catalysts. J Nat Gas Chem 16:92–99
Li X, Shen B, Guo Q, Gao J (2007) Effects of large pore zeolite additions in the catalytic pyrolysis catalyst on the light olefins production. Catal Today 125:270–277. doi:10.1016/j.cattod.2007.03.021
Liu C, Gao X, Zhang Z, Zhang H, Sun S, Deng Y (2004) Surface modification of zeolite Y and mechanism for reducing naphtha olefin formation in catalytic cracking reaction. Appl Catal A Gen 264:225–228. doi:10.1016/j.apcata.2003.12.048
Liu D, Choi WC, Lee CW, Kang NY, Lee YJ, Shin C-H, Park YK (2011) Steaming and washing effect of P/HZSM-5 in catalytic cracking of naphtha. Catal Today 164:154–157. doi:10.1016/j.cattod.2010.10.091
López-Isunza F, Moreno-Montiel N, Quintana-Solórzano R, Moreno-Mayorga JC, Hernández-Beltrán F (2001) Modelling diffusion, cracking reactions and deactivation in FCC Catalysts. In: Froment GF, Waugh KC (eds) Studies in surface science and catalysis, vol 133. Elsevier, pp 509–514. doi:10.1016/S0167-2991(01)82004-8
Lu Y, He M, Song J, Shu X (2001) Active site accessibility of resid cracking catalysts. In: GehrkOccellie ML, Connor PO (eds) Studies in surface science and catalysis, vol 134. Elsevier, pp 209–217. doi:10.1016/S0167-2991(01)82321-1
Ma’adhah A, Abul-Hamayel M, Aitani A, Ino T, Okuhara T (2000) Down-flowing FCC reactor increase propylene gasoline make. Oil Gas J 98:66–70
Maadhah AG, Fujiyama Y, Redhwi H, Abul-Hamayel M, Aitani A, Saeed M, Dean C (2008) A new catalytic cracking process to maximize refinery propylene. Arabian J Sci Eng 33:17–28
Mann R, El-Nafaty UA (1996) Probing internal structures of FCC catalyst particles: from parallel bundles to fractals. In: M. Absi-Halabi JB, Stanislaus A (eds) Studies in surface science and catalysis, vol 100. Elsevier, pp 355–364. doi:10.1016/S0167-2991(96)80035-8
Mao RLV (2008) Co-catalysts for hybrid catalysts, hybrid catalysts comprising same, monocomponent catalysts, methods of manufacture and uses thereof
Mao RLV, Al-Yassir N, Nguyen DTT (2005) Experimental evidence for the pore continuum in hybrid catalysts used in the selective deep catalytic cracking of n-hexane and petroleum naphthas. Microporous Mesoporous Mater 85:176–182. doi:10.1016/j.micromeso.2005.05.050
Martınez C, Verboekend D, Perez-Ramırez J, Corma A (2013) Stabilized hierarchical USY zeolite catalysts for simultaneous increase in diesel and LPG olefinicity during catalytic cracking Catal. Sci Technol 3:972–981
Meng X, Xu C, Gao J, Li L (2005) Studies on catalytic pyrolysis of heavy oils: reaction behaviors and mechanistic pathways. Appl Catal A General 294:168–176. doi:10.1016/j.apcata.2005.07.033
Meng X, Xu C, Gao J, Zhang Q (2004) Effect of catalyst to oil weight ratio on gaseous product distribution during heavy oil catalytic pyrolysis. Chem Eng Process Process Intensif 43:965–970. doi:10.1016/j.cep.2003.09.003
Meusinger J, Corma A (1996) Influence of zeolite composition and structure on hydrogen transfer reactions from hydrocarbons and from hydrogen. J Catal 159:353–360. doi:10.1006/jcat.1996.0097
Miale JN, Chen NY, Weisz PB (1966) Catalysis by crystalline aluminosilicates: IV. Attainable catalytic cracking rate constants, and superactivity. J Catal 6:278–287. doi:10.1016/0021-9517(66)90059-5
Mochizuki H, Yokoi T, Imai H, Watanabe R, Namba S, Kondo JN, Tatsumi T (2011) Facile control of crystallite size of ZSM-5 catalyst for cracking of hexane. Microporous Mesoporous Mater 145:165–171. doi:10.1016/j.micromeso.2011.05.011
Möller KP, Böhringer W, Schnitzler AE, van Steen E, O’Connor CT (1999) The use of a jet loop reactor to study the effect of crystal size and the co-feeding of olefins and water on the conversion of methanol over HZSM-5. Microporous Mesoporous Mater 29:127–144. doi:10.1016/S1387-1811(98)00326-6
Nexant (2009) Propylene technology: the next generation
Nishiyama N, Kawaguchi M, Hirota Y, Van Vu D, Egashira Y, Ueyama K (2009) Size control of SAPO-34 crystals and their catalyst lifetime in the methanol-to-olefin reaction. Appl Catal A Gen 362:193–199. doi:10.1016/j.apcata.2009.04.044
O’Connor P, Imhof P, Yanik SJ (2001) Catalyst assembly technology in FCC. Part I: a review of the concept, history and developments. In: GehrkOccellie ML, Connor PO (eds) Studies in surface science and catalysis, vol 134. Elsevier, pp 299–310. doi:10.1016/S0167-2991(01)82329-6
O’Connor P, Humphies AP (1993) Accessibility of functional sites in FCC. Prepr Am Chem Soc Div Pet Chem 38:598–603
Ouyang FS, Weng HX (2007) Investigation of the operating conditions on decreasing FCC gasoline olefin content by GOR-Q catalyst. Petrol Sci Technol 25:399–409. doi:10.1080/10916460600803603
Paparatto G, Moretti E, Leofanti G, Gatti F (1987) Toluene ethylation on ZSM zeolites. J Catal 105:227–232. doi:10.1016/0021-9517(87)90021-2
Parthasarathi RS, Alabduljabbar S (2014) HS-FCC High-severity fluidized catalytic cracking: a newcomer to the FCC family. Appl Petrochem Res 4:441–444. doi:10.1007/s13203-014-0087-5
Peng P (1998) New processes for LCO upgrading from deep catalytic cracking. Fuel Energy Abstr 39:339, 398/03601. doi:10.1016/S0140-6701(98)93596-2
Perego C, Millini R (2013) Porous materials in catalysis: challenges for mesoporous materials. Chem Soc Rev 42:3956–3976. doi:10.1039/C2CS35244C
Platon A, Thomson W (2005) Low-temperature test reaction for hydride transfer on solid acid catalysts. Catal Lett 101:15–20. doi:10.1007/s10562-005-3741-9
Radcliffe C (2007) The FCC unit as a propylene source catalysts additives. http://wwwdigitalrefiningcom/article_1000312pdf 14
Rana MS, Sámano V, Ancheyta J, Diaz JAI (2007) A review of recent advances on process technologies for upgrading of heavy oils and residua. Fuel 86:1216–1231. doi:10.1016/j.fuel.2006.08.004
Rase HF (2000) Handbook of commercial catalysts: heterogeneous catalysts. CRC Press LLC, New York
Ren T, Patel M, Blok K (2006) Olefins from conventional and heavy feedstocks: energy use in steam cracking and alternative processes. Energy Fuels 31:425–451
Sadeghbeigi R (2000) Fluid catalytic cracking handbook: design, operation and troubleshooting of FCC facilities, 2nd edn. Gulf Publishing Company, Austin
Sertić-Bionda K, Kuzmić V, Jednačak M (2000) The influence of process parameters on catalytic cracking LPG fraction yield and composition. Fuel Process Technol 64:107–115. doi:10.1016/S0378-3820(99)00124-1
Shao Q, Wang P, Tian H, Yao R, Sun Y, Long J (2009) Study of the application of structural catalyst in naphtha cracking process for propylene production. Catal Today 147:S347–S351. doi:10.1016/j.cattod.2009.07.056
Shaw to provide Deep Catalytic Cracking technology for Punjab refinery (2008) Focus on catalysts 2008:5. doi:10.1016/S1351-4180(08)70223-X
Siddiqui MAB, Aitani AM, Saeed MR, Al-Yassir N, Al-Khattaf S (2011) Enhancing propylene production from catalytic cracking of Arabian Light VGO over novel zeolites as FCC catalyst additives. Fuel 90:459–466. doi:10.1016/j.fuel.2010.09.041
Silverman LD, Winkler WS, Tiethof JA, Witoshkin A (1986) Matrix effects in catalytic cracking. Paper presented at the NPRA Meeting, Los Angeles, California, March 23–26, 1986
Soni D, Rao MR, Saidulu G, Bhattacharyya D, Satheesh VK (2009) Catalytic cracking process enhances production of olefins, vol Q4
Stockwell DM, Liu X, Nagel P, Nelson PJ, Gegan TA, Keweshan CF (2004) Distributed Matrix Structures—novel technology for high performance in short contact time FCC. In: Occelli M (ed) Studies in surface science and catalysis, vol 149. Elsevier, pp 257–285. doi:10.1016/S0167-2991(04)80768-7
Tago T, Konno H, Nakasaka Y, Masuda T (2012) Size-controlled synthesis of nano-zeolites and their application to light olefin synthesis. Catal Surv Asia 16:148–163
Teimouri Sendesi SM, Towfighi J, Keyvanloo K (2012) The effect of Fe, P and Si/Al molar ratio on stability of HZSM-5 catalyst in naphtha thermal-catalytic cracking to light olefins. Catal Comm 27:114–118. doi:10.1016/j.catcom.2012.06.013
Teng J, Wang R, Xie Z, Gan Y (2008) New Olefin Production Technologies. In: SINOPEC SRIPT. 29 June–3 July 2008
Triantafillidis CS, Evmiridis NP, Nalbandian L, Vasalos IA (1999) Performance of ZSM-5 as a fluid catalytic cracking catalyst additive: effect of the total number of acid sites and particle size. Ind Eng Chem Res 38:916–927. doi:10.1021/ie980395j
Von Ballmoos R, Hayward C-MT (1991) Matrix vs zeolite contributions to the acidity of fluid cracking catalysts. In: Öhlmann G, Fricke R (eds) Studies in surface science and catalysis, vol 65. Elsevier, pp 171–183. doi:10.1016/S0167-2991(08)62905-5
Wang DZ (1996) Deep catalytic cracking for production of light olefins from heavy feedstocks. Appl Catal A Gen 141:N4–N5. doi:10.1016/0926-860X(96)80111-6
Yan HT, Mao RLV (2010) Hybrid catalysts used in the Catalytic Steam Cracking process (CSC): influence of the pore characteristics and the surface acidity properties of the ZSM-5 zeolite-based component on the overall catalytic performance. Appl Catal A Gen 375:63–69
Zai-Ting L, Chao-Gang X, Jiu-shun Z, Zhi-Gang Z (2002) Olefin production technology with adjustable propylene/ethylene ratio by catalytic cracking route. 1–5 Sept 2002
Zhao G, Teng J, Xie Z, Jin W, Yang W, Chen Q, Tang Y (2007) Effect of phosphorus on HZSM-5 catalyst for C4-olefin cracking reactions to produce propylene. J Catal 248:29–37. doi:10.1016/j.jcat.2007.02.027
Zhao X, Harding RH (1999) ZSM-5 additive in fluid catalytic cracking. 2 Effect of hydrogen transfer characteristics of the base cracking catalysts and feedstocks. Ind Eng Chem Res 38:3854–3859. doi:10.1021/ie990180p
Zhao X, Roberie TG (1999) ZSM-5 additive in fluid catalytic cracking. 1 Effect of additive level and temperature on light olefins and gasoline olefins. Ind Eng Chem Res 38:3847–3853. doi:10.1021/ie990179q
Zhu X, Liu S, Song Y, Xu L (2005) Catalytic cracking of C4 alkenes to propene and ethene: influences of zeolites pore structures and Si/Al2 ratios. Appl Catal A Gen 288:134–142. doi:10.1016/j.apcata.2005.04.050
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution License which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
About this article
Cite this article
Akah, A., Al-Ghrami, M. Maximizing propylene production via FCC technology. Appl Petrochem Res 5, 377–392 (2015). https://doi.org/10.1007/s13203-015-0104-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13203-015-0104-3