Abstract
The modification of commercial ultra-stable Y zeolites using citric acid and phosphoric acid was investigated systematically via a L18(38) orthogonal experiment. The pore structure, acid property and crystal structural of modified USY zeolites were characterized by a variety of means such as N2 adsorption–desorption, Fourier transform infrared spectroscopy, NH3-temperature programmed desorption and X-ray diffraction. The optimal modification condition is found to be that the volume ratio of citric acid (0.3 mol/L) and phosphoric acid (0.3 mol/L) is 1.0, and the operation is performed at 100 °C for 6 h. The as-synthesized sample presents an increased secondary pore volume up to 0.207 cm3/g which accounts for 42.9 % of the total pore volume, and appropriate acidity distribution as well as good crystallinity. In addition, the USY obtained by 1.0 L scale-up modification possesses a secondary pore volume of 0.210 cm3/g which accounts for 42.4 % of the total pore volume, showing no obvious scale-up effects. Furthermore, the hydrothermal stability of the modified samples meets the requirements of commercial catalysts for hydrocracking. Performance evaluation was carried out on a 200 mL fixed-bed single stage hydrogenation unit using Daqing VGO as feedstock. The 140–370 °C middle distillate yield is 66.09 %, and middle distillate selectivity can reach up to 80.45 %. Compared with commercial catalyst, the yield and selectivity are increased by 5.67 and 4.07 %, respectively.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In recent years, the demand for high quality middle distillate has been increasing. Hydrocracking of hydrocarbons continues to act as a typical process in the petroleum refining industry for upgrading vacuum distillates and residues to produce valuable gasoline or diesel fuels [1–3]. Y and ultra-stable Y (USY) zeolites have been commonly applied in increasing middle distillate yield as the hydrocracking additives due to their high specific surface area and tunable acid properties. Well-developed secondary pore structure, appropriate acid distribution as well as high crystallinity are essential to an ideal hydrocracking catalyst. However, the performance of commercial USY is unsatisfying due to the lack of developed mesopore volume. Moreover, high acid strength and density also lead to serious secondary cracking. Therefore, the modification of USY zeolites is of great significance [4].
Dealumination of Y zeolites improves its thermal and hydrothermal stability, hydrophobicity and creates abundant mesopores [5, 6]. Typical routes for creating mesopore of Y zeolites include hydrothermal method [7], chemical method [8–13] and combined methods. Steaming of Y zeolites is the mostly used method for dealumination to prepare USY zeolites. The hydrothermal modification of the zeolites framework proceeds with the hydrolysis of the Al–O–Si bonds and thereby slightly destroys its crystal structure through shaping mesoporosity [14], leaving aluminum with different forms of extra-framework alumina (EFAL) in the zeolites structure [15]. Extra-framework siliceous admixtures are formed by the decomposition of Al-rich and Si-rich regions of the framework, resulting in disproportionation into an Al-rich aluminosilicate and silica gel by steaming treatment [16]. However, the pore size and acid distribution of the zeolites with hydrothermal treatment are unreasonable for meeting the needs of hydrocracking [17, 18]. Dealumination of USY zeolites via SiCl4 induces a good distribution of Al and Si atoms in the framework although the dealumination process could generate some oxygen and chlorine species that cannot be removed completely. Also, the crystallinity significantly decreased under the harsh reaction condition. Zeolites treated by (NH4)2SiF6 possesses high crystallinity while it has little amount of mesopores [9, 19]. Modification using SiCl4, (NH4)2SiF6 or NH4F not only undergoes harsh conditions, but also produces many environmental pollutants, such as Cl−, F−. Many researchers also modify Y zeolites via inorganic acids, such as hydrochloric acid [20], nitric acid [21] and phosphoric acid [22]. The amount of aluminium removed from the framework is found to be proportional to the hydrogen ion concentration of the acid solution. Dealumination does not occur when the pH of the acid solution is higher than 2.30, while complete dealumination is observed at pH values that lower than 0.46 [20]. EDTA coordination reaction is of relative high selectivity to dealumination. However, the crystals of as-prepared zeolites are not uniform [23]. In addition, its operating condition is strict and now only available at laboratory scale.
In order to integrate mesopore, crystallinity and acid properties simultaneously, combined modification method is adopted. Gomes et al. [24]. Reported a combined modification of USY zeolites with sulphuric and phosphoric combined acid. H2SO4 removes the EFAL located in supercavities without attacking the zeolitic framework. H3PO4 incorporates P in two quite distinct ways: as a monomeric phosphate associated to framework aluminium atoms (for low EFAL concentrations) and as a polymeric phosphate originated from the reaction of EFAL with H3PO4 (for high EFAL concentrations). The mesopores content increases without obvious decrease of crystallinity. However, H2SO4 will cause a serious corrosion when it is applied in industry. Compared with inorganic acids, organic acids are more potential because they could modify the USY zeolites in moderate reaction conditions thus no obvious defects are observed in the modified products [4, 25]. Previous researches [26–28] have shown that citric acid, acting as an organic ligand, demonstrates excellent pore-forming ability.
Herein, to explore excellent routes, we modified commercial USY zeolites by using citric acid and phosphoric combined acid. Citric acid removed EFAL via complexation, phosphoric acid maintains good crystallinity and adjust the acid property. We designed a L18(38) orthogonal experiment to find out the optimum modification condition. The effects of the various factors on the USY zeolites were tentatively investigated. Furthermore, the crystal structure, physical adsorption property, stability and acid property were also investigated by XRD, N2 adsorption–desorption, pyridine adsorption FT-IR and NH3-TPD.
Experimental
Orthogonal experiment design
During the modification, an appropriate stirring rate of 300 r/min and a liquid–solid ratio of 10 mL/g were selected. Concentration of citric acid (A), concentration of phosphoric acid (B), liquid–liquid ratio of citric acid to phosphoric acid (C), order of feed (D), reaction time (E) and reaction temperature (F) were all taken into consideration. Three levels were selected for each factor based on experience and literature [22, 24, 25] (Table 1). Volume of secondary pore (V) and the proportion of medium acid (Y) were adopted as criteria and thus orthogonal layout was given in the following form (Table 2). Well-developed secondary pore structure and appropriate acid property are crucial to high selectivity of hydrocracking catalysts to middle distillate. Mesopores act as smooth channels for the diffusion of middle distillate molecule. Hence, they can avoid the secondary cracking, improve middle distillate selectivity and enhance yield of liquid oil. In addition, high acidity tends to cause coking, which leads to catalyst deactivation. The catalyst deactivation will cause a reduction of product selectivity and quality. Thus it is reasonable that the volume of secondary pore and the proportion of medium acid are adopted as criteria.
Sample preparation
First, citric acid solution and phosphoric acid solution with different concentrations were prepared. Then a fixed quantity of 12 g commercial USY (CUSY) zeolites was put into a 250 mL three-neck flask. Subsequently a certain amount of citric acid solution and phosphoric solution were added. The reaction was completed in a water bath at certain temperature for a certain time according to the orthogonal experiment layout. Afterwards, the product was filtrated and washed with water. At last the product was obtained after dried in a 110 °C oven overnight. A four times scale-up experiment was carried out with same procedure in a 1 L reactor.
Sample characterization
Nitrogen-sorption measurements were performed on a TriStar 3000 analyzer (Micromeritics, USA) to obtain specific surface area and pore structure parameters of the as-prepared samples. The total surface area was calculated using BET (Brunauer–Emmett–Teller) method. The mesopore surface area, mesopore volumes and pore size distribution were obtained from the desorption branch by a BJH (Barret–Joyner–Halenda) method. Si/Al ratio, cell parameters and crystallinity were characterized by X-ray diffraction (XRD) analysis (X’Pert PRO MPD, Holland). The acidy property of the sample was measured by FT-IR (Nicolet 6700, U.S.A.) using pyridine as probe molecule. NH3-temperature programmed desorption (NH3-TPD) characterization was carried out on CHEMBET-3000 TPR/TPD Chemisorption analyzer (Quatachrome Instrument, U.S.A.). NH3-TPD curves were performed in the range of 50–700 °C with a heating rate of 15 °C per minute. The ammonia was adsorbed at room temperature while it was desorbed at 120 °C for 1 h in flowing pure nitrogen.
Results and discussion
Screening of optimum condition
The modification conditions and results are listed in Table 3. The significance of each factor is judged according to R value. The factor with high R value is considered to be major factor to the criterion. According to R, the rank of the significance for secondary pore volume and proportion of medium acid are B > F > A > E(C) > D and D > B > A > E > C > F, respectively. It is well known that higher content of secondary pore and medium acid are more favorable to hydrocracking. Hence the factors with the highest Vj and Yj are chosen as the optimum condition. According to Vj and Yj listed in Table 3, the optimum conditions for secondary pore and proportion of medium acid are B3F3A2C2E1D3 and D3B2A3E2C2F3, respectively. Given this, we determine the optimum modification condition: D3B3A2E2C2F3. However, if citric acid and phosphoric acid are added into the reaction simultaneously (D3), the crystallinity of the as-prepared samples will decrease significantly. So the second-best D1 is selected. Finally the optimal condition for combined modification of USY zeolites is D1B3A2E2C2F3. That is to say the citric acid (0.3 mol/L) and phosphoric acid (0.3 mol/L) are orderly added with a volume ratio of 1.0, and the operation is performed at 100 °C for 6 h.
Sample characterization result and discussion
The modified USY zeolites (MUSY) was prepared at the optimal modification condition. A four times scale-up modified USY zeolites (SMUSY) was also prepared in a 1 L autoclave with same procedure.
Surface area and pore size distribution of modified USY
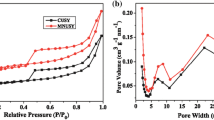
N2 adsorption–desorption isotherms determined at 77 K and the pore size distribution of the prepared samples are shown in Figs. 1 and 2. All samples present a type IV adsorption isotherm with a H2 hysteresis loop ranging from relative pressure of 0.43 to 1.0, which is the characteristic of abundant mesopores. In addition, the specific surface area of MUSY increases from 641 to 667 and 658 m2/g while the mesopore volume increases from 0.168 cm3/g to 0.207 and 0.210 cm3/g (Table 4) after modification, respectively. The EFAL is removed by citric acid via coordination effect while some framework aluminum (FAL) is removed by phosphoric acid, leading to the increase of specific surface area and volume. The pore size distribution (Fig. 2) shows that modified USY zeolites possess intensive distribution of secondary pores which are centralized at about 8.0 and 23 nm.
Acid characterization of modified USY
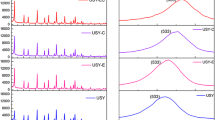
The acid properties of the samples were characterized by pyridine adsorption FT-IR and NH3-TPD. It is found that there are three bands for each sample in the FT-IR spectra (Fig. 3). The bands at 1,442 and 1,548 cm−1 represent the characteristic adsorption of pyridine on Lewis (L) and Brøsted (B) acidic sites, respectively. While band at 1,490 cm−1 is assigned to the combined effect of B and L acidic sites [29]. Compared with CUSY, MUSY and SMUSY present higher intensity at 1,442 cm−1, indicating the increment of L acidic sites for the modified USY. Besides, the weaker peaks assigned to B acid demonstrate the decrease of B acid sites. The acid strength was measured by NH3-TPD. All curves present two peaks at 200 and 450 °C (Fig. 4). Interestingly, the 200 °C peaks of MUSY and SMUSY shift to higher temperature, indicating that the acid strength of weak acid increased. According to the peak area, the acid amount of both strong acid and weak acid decreases. However, the amount of weak acid decreases much more than medium acid, leading to an increase of proportion of medium acid. There is no obvious difference between MUSY and SMUSY, which suggests that the amplification effect is negligible during the modification process.
X-ray diffraction characterization
The crystal structure parameters of modified USY are listed in the Table 5. It shows that the Si/Al ratios of the modified samples are greater than that of CUSY. The cell parameters become smaller and crystallinity decreases after modification. The improvement of Si/Al ratio is caused by the dealumination and silicon reinsertion. Moreover, the insertion of phosphate-hydrolyzed phosphoric acid should also be considered. Because of the connection between aluminum atoms and phosphorus atoms through oxygen bridge bonds, the increase of Si/Al ratio may include the contribution of P/Al ratio. Owing to the replacement of aluminum by silicon or phosphorus, unit cell parameters become smaller since both Si–O and P–O bond length are shorter than Al–O. The significant decrease in crystallinity is ascribed to the local skeleton collapse caused by dealumination.
Hydrothermal stability characterization
The modified USY zeolites were treated at 600 °C with the existence of vapor for 4 h. Then the specific surface area and pore structure parameters were measured to study their hydrothermal stability. The results listed in Table 6 demonstrate that the specific surface area and volume of micropores decrease after hydrothermal treatment, while the specific surface area and volume of mesopores increase. This is mainly because the micropores are destroyed after a long-term treatment with steam and the mesopores form with the connection of micropores. Furthermore, the steam can also remove the framework alumina at high temperature, giving rise to mesopores. Generally, the modified USY zeolites has a good hydrothermal stability under 600 °C which is much higher than the hydrocracking reaction temperature.
Evaluation of catalyst
According to the 75.9 % conversion of >350 °C feedstock (Table 7), SMUSY exhibits excellent hydrocracking performance. The 140–370 °C middle distillate yield of hydrocracking product is 66.09 %, and at the same time, the selectivity to middle distillate can reach up to 80.45 %. Compared with CUSY, the yield and selectivity are increased by 5.67 and 4.07 %, respectively. The results demonstrate that combined modification of ultra-stable Y zeolites using citric acid and phosphoric acid can meet the need of productive middle distillate in the industrial unit, which predicts it may be a potential industrialized method of Y zeolites modification.
Conclusion
A combined modification of commercial USY zeolites by using citric acid and phosphoric acid has been successfully developed. On the basis of L18(38) orthogonal experiment, the optimal modification conditions are recommended in this work. The modified USY zeolites prepared under the optimum technological conditions presents an enhanced secondary pore volume and appropriate acid distribution as well as good crystallinity. There is no obvious difference in properties of samples prepared in the conditions of the 250 mL and the 1 L scale experiment, exhibiting a good scalability. In addition, the amount and strength of medium acid, as well as the hydrothermal stability of the modified USY zeolites are superior compared with its pristine counterpart. Thus the modified USY zeolites present an excellent middle distillate selectivity when it is used in hydrocracking.
References
Cui Q-Y, Zhou Y-S, Wei Q, Yu G-L, Zhu L (2013) Performance of Zr-and P-modified USY-based catalyst in hydrocracking of vacuum gas oil. Fuel Process Technol 106:439–446
Francis J, Guillon E, Bats N, Pichon C, Corma A, Simon LJ (2011) Design of improved hydrocracking catalysts by increasing the proximity between acid and metallic sites. Appl Catal A 409:140–147
Ishihara A, Itoh T, Nasu H, Hashimoto T, Doi T (2013) Hydrocracking of 1-methylnaphthalene/decahydronaphthalene mixture catalyzed by zeolite-alumina composite supported NiMo catalysts. Fuel Process Technol 116:222–227
Chang X-W, He L-F, Liang H-N, Liu X-M, Yan Z-F (2010) Screening of optimum condition for combined modification of ultra-stable Y zeolites using multi-hydroxyl carboxylic acid and phosphate. Catal Today 158:198–204
Mcdaniel C, Maher P (1968) Molecular Sieves. Society of Chemical Industry, London 186
Sasaki Y, Suzuki T, Takamura Y, Saji A, Saka H (1998) Structure analysis of the mesopore in dealuminated zeolite Y by high resolution tem observation with slow scan CCD camera. J Catal 178:94–100
Wang Q-L, Giannetto G, Torrealba M, Perot G, Kappenstein C, Guisnet M (1991) Dealumination of zeolites II. Kinetic study of the dealumination by hydrothermal treatment of a NH4 NaY zeolite. J Catal 130:459–470
Corma A, Fornes V, Rey F (1990) Extraction of extra-framework aluminium in ultrastable Y zeolites by (NH4)2SiF6 treatments: I. Physicochem Charact Appl Catal 59:267–274
López-Fonseca R, De Rivas B, Gutiérrez-Ortiz J, Aranzabal A, González-Velasco JR (2003) Enhanced activity of zeolites by chemical dealumination for chlorinated VOC abatement. Appl Catal B 41:31–42
Panov AG, Gruver V, Fripiat JJ (1997) Fluorination of USY and modification of its catalytic properties. J Catal 168:321–327
Kerr GT (1968) Chemistry of crystalline aluminosilicates. V. Preparation of aluminum-deficient faujasites. J Phys Chem 72:2594–2596
Datka J, Kolidziejski W, Klinowski J, Sulikowski B (1993) Dealumination of zeolite Y by H4EDTA. Catal Lett 19:159–165
Datka J, Klinowski J, Sulikowski B (1994) Dealumination of zeolite Y by H 4 EDTA: further comments. Catal Lett 25:403–404
Hernández-Beltrán F, Moreno-Mayorga JC, De Lourdes Guzmán-Castillo MA, Navarrete-Bolaños J, González-González M, Handy BE (2003) Dealumination–aging pattern of REUSY zeolites contained in fluid cracking catalysts. Appl Catal A: Gen 240:41–51
Gore KU, Abraham A, Hegde SG, Kumar R, Amoureux J-P, Ganapathy S (2002) 29Si and 27Al MAS/3Q-MAS NMR studies of high silica USY zeolites. J Phys Chem B 106:6115–6120
Lutz W, Toufar H, Heidemann D, Salman N, Rüscher C, Gesing T, Buhl J-C, Bertram R (2007) Siliceous extra-framework species in dealuminated Y zeolites generated by steaming. Microporous Mesoporous Mater 104:171–178
Dong S-T, Li X-W, Li D-D, Shi Y-H, Nie H, Kang X-H (2002) Study on the formation of mesopore during hydrothermal dealumination of Y zeolite. Acta Physico-chimica Sinica 18:201–206
Yoshida A, Inoue K, Adachi Y (1991) Hydrothermal stability of US-Ex. Zeolites 11:223–231
Matharu A, Gladden L, Carr S (1995) Characterisation and catalytic properties of dealuminated zeolite-Y: a comparison of ammonium hexafluorosilicate and hydrothermal treatments. Stud Surf Sci Catal 94:147–154
Lee EF, Rees LV (1987) Dealumination of sodium Y zeolite with hydrochloric acid. J Chem Soc, Faraday Trans 1: Phys Chem Condens Phases 83:1531–1537
Yan Z-M, Ma D, Zhuang J-Q, Liu X-C, Liu X-M, Han X-W, Bao X-H, Chang F-X, Xu L, Liu Z-M (2003) On the acid-dealumination of USY zeolite: a solid state NMR investigation. J Mol Catal A: Chem 194:153–167
Panneerselvam P, Thinakaran N, Thiruvenkataravi K, Palanichamy M, Sivanesan S (2008) Phosphoric acid modified-Y zeolites: a novel, efficient and versatile ion exchanger. J Hazard Mater 159:427–434
Dwyer J, Fitch F R, Machado F, Qin G, Smyth S M, and Vickerman J C (1981) The surface composition of dealuminated zeolites. J Chem Soc, Chem Commun: 422–424
Leiras Gomes A, Falabella S, Aguiar E, Cabral Menezes S, Cardoso D (1997) Influence of combined acid treatment on physico-chemical characteristics of ultrastable zeolite Y and on its catalytic properties in the disproportionation of ethylbenzene. Appl Catal A 148:373–385
Liu X-M, Yan Z-FY (2001) Optimization of nanopores and acidity of USY zeolite by citric modification. Catal Today 68:145–154
Xie Z-K, Chen Q-L, Zhang C-F, Bao J-Q, Cao Y-H (2000) Influence of citric acid treatment on the surface acid properties of zeolite beta. J Phys Chem B 104:2853–2859
Fan Y, Bao X-J, Lin X-Y, Shi G, Liu H-Y (2006) Acidity adjustment of HZSM-5 zeolites by dealumination and realumination with steaming and citric acid treatments. J Phys Chem B 110:15411–15416
Fan Y, Lin X-Y, Shi G, Liu H-Y, Bao X-J (2007) Realumination of dealuminated HZSM-5 zeolite by citric acid treatment and its application in preparing FCC gasoline hydro-upgrading catalyst. Microporous Mesoporous Mater 98:174–181
Lercher J, Rumplmayr G (1986) Controlled decrease of acid strength by orthophosphoric acid on ZSM5. Appl catal 25:215–222
Acknowledgments
This work was financially supported by PetroChina Refinery Catalyst Key Project (2010E-1903).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution License which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
About this article
Cite this article
Li, X., Qiao, K., He, L. et al. Combined modification of ultra-stable Y zeolites via citric acid and phosphoric acid. Appl Petrochem Res 4, 343–349 (2014). https://doi.org/10.1007/s13203-014-0070-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13203-014-0070-1