Abstract
Calculating the minimum miscibility pressure (MMP) between crude oil and carbon dioxide (CO2) is critical for optimizing injection parameters, designing schemes, and predicting production capacity in CO2 injection projects for enhancing oil recovery. However, an accurate approach for obtaining this parameter is not yet established. In order to tackle this issue, a novel approach is suggested, based on the original cell-to-cell model, to determine the MMP and the 97% oil recovery rate as the standard. Using the volume-transformed Peng-Robinson equation of state enhances the precision of fluid volume estimation, as it mainly relies on predicting fluid volume within each cell. Furthermore, to ensure a precise estimation of the ultimate oil recovery rate, it is imperative to employ a total cell count of 500 in all simulations to avoid the problem of numerical dispersion. Finally, a second-order polynomial equation more accurately predicts the infinite-cell oil recovery factor. The accuracy of the modified model is verified by comparing MMP values from five oil and gas systems in the literature. The computational results of the modified multiple-mixing-cell (MMC) approach exhibit a higher level of concordance with the MMPs in the literature. The average relative error is less than 3.96%. The improved MMC algorithm can quickly determine the miscibility mechanism and visually represent the dynamic miscibility process involving multiple oil-gas contacts in a slim tube. This study provides a theoretical and practical basis for addressing the critical scientific issues of CO2-safe storage technology.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
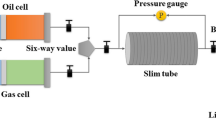
In recent years, under global efforts to promote carbon neutrality, carbon capture, utilization, and storage(CCUS) technology has brought opportunities for rapid development and attracted great attention. The CO2 injection process is an effective method of CCUS, which can enhance oil recovery and store the greenhouse gas CO2 in depleted reservoirs (Sun et al. 2023; Li et al. 2022). The accurate prediction of oil-gas minimum miscibility pressure (MMP) is essential for parameter optimization, program design, and production prediction of CO2 injection projects. Most recently, the methods for determining oil-gas MMP are experimental methods, empirical correlations, and numerical simulations (Dindoruk et al. 2021). The experimental methods are mainly Slim Tube (Ali et al. 2022), Vanishing Interfacial Tension (VIT) (Orr et al. 2007; Mutailipu et al. 2019), Multiple Contact Method (MCM) (Shojaei et al. 2012), and Rising Bubble Apparatus (RBA) (Elsharkawy et al. 1996; Zhang et al. 2019). In the petroleum industry, the slim-tube test is widely acknowledged as the industry criterion for estimating MMP (Zhang et al. 2019), mainly due to its ability to represent the complicated interactions between phase behavior and fluid flow in pores. However, the slim-tube test is time-consuming and high-cost. When the mechanism of miscible gas displacement is condensing displacement (CD) or vaporizing displacement (VD), MMP measured by MCM has high accuracy. When the miscible displacement mechanism combines vaporizing and condensing displacement (VCD), the MMP determined by the MCM is higher than the actual value. Other experimental methods (e.g., RBA and VIT) cannot obtain reliable MMP because the mechanism of miscible gas displacement in most oilfields is VCD. Besides experimental methods, some empirical correlation equations for determining MMP are established by fitting experimental data points within a specific range of reservoir temperature and properties of petroleum and CO2 (Liao et al. 2014). Compared with the experimental method, the empirical correlation methods are simple, fast, and low-cost. However, more minor changes in reservoir conditions can produce more significant errors.
Due to the disadvantages of experimental and empirical correlation methods, many attempts have been made in the past decades to establish the computational algorithms by equations of state (EOS) for determining the MMP. In contrast to experimental methods, computational methods offer advantages in terms of speed and convenience. At present, there exist three principal computational methodologies (Dindoruk et al. 2021): one-dimensional (1D) slime-tube simulation (Stalkup 1990; He et al. 2019), the method of characteristics (MOC) (Wang and Orr 1997; Ahmadi et al. 2011a), and the multiple-mixing-cell model (MMC) (Ge et al. 2021). The 1D slim-tube model is a numerical simulation of the flow in the pore medium during a slim-tube experiment. Analogous to the slim-tube test, the MMP calculation involves analyzing the breaking point of the recovery rate against the injection pressure curve. However, the fine-mesh simulation is subject to numerical dispersion, which results in inaccuracies in the prediction of MMP. The simulation is conducted at varying pressures and grid sizes to eliminate the numerical dispersion (He et al. 2019). This approach allows for extrapolation to achieve zero-dispersion recovery and determination of the MMP. Compared with other computational methodologies, the 1D slim-tube simulation is characterized by its tedious, mechanized, and time-consuming nature. The MOC for determining the MMP relies on analytically solving the 1D nondispersive flow equations (Wang and Orr 1997). This solution enables the identification of a sequence of intersecting critical tie-lines. When the pressure is elevated, the pressure at which the minimum key tie-line length becomes zero, which is referred to as the MMP. Nevertheless, in the case of gases injected with several components, the MOC can converge towards an incorrect series of key tie-lines, thereby presenting a potential limitation of this method (Ahmadi et al. 2011a; Dindoruk et al. 2021).
The MMC (or cell-to-cell) model was initially suggested by Metcalfe et al. (1973) to investigate the mechanism of miscibility. The fundamental concept underlying the MMC model involves the repeated interaction of oil and gas within a single cell or several cells to generate new equilibrium compositions. When the mechanism of miscibility is either VD or CD, the MMC algorithm can accurately determine a dependable MMP (Dindoruk et al. 2021). However, it is worth noting that most oilfield miscibility displacements exhibit VCD (Jaubert et al. 1998a and 1998b). Hence, the earlier MMC model cannot provide a reliable MMP. In recent years, different versions of the MMC model have been proposed to improve the accuracy of predicting MMP. In the literature, two prominent models exist: the Ahmadi-MMC technique, introduced by Ahmadi et al. (2011b), and the Jaubert-MMC method, proposed by Jaubert et al. (1998a). The Ahmadi-MMC model combines the MMC with the MOC theory, determining the MMP based on a minimum key tie-line length of zero. Due to the absence of the physical mechanism of fluid flow in the Ahmadi-MMC model, determining the volume of individual cells and mixed fluid is unattainable. Hence, the model fails to provide oil recovery data and is unsuitable for exclusive application in the three-phase displacement scenario (Li et al. 2019). The Jaubert-MMC method is a simplified version of the 1D slim-tube model, achieved by replacing the grid cells with mixing cells (Jaubert et al. 1998a). One of the advantages of this model is that it requires only a single simulation computation at a sufficiently large number of cells under a certain pressure. This advantage allows for determining oil recovery values for different numbers of cells and facilitates linear extrapolation to estimate the oil recovery rate for infinite cells. This model’s cell-to-cell transfer of excessive fluid volume can be attributed to a natural physical event observed during the gas displacement. Hence, it is possible to expand this model to encompass the scenario of three-phase displacement (Zhao et al. 2006; Moghaddam and Dehaghani 2017). The accuracy of the methods adopted to calculate the MMP with the 97% oil recovery criterion is comparatively lower than alternative approaches (Moghaddam and Dehaghani 2017). A few studies focus on utilizing the zero minimum key tie-line length criterion to improve the accuracy of calculating MMP (Yang et al. 2020).
This work proposes a new approach for determining the MMP based on the Jaubert-MMC model, with the standard of 97% oil recovery rate. Three improvements are performed to improve the accuracy of determining the MMP. Firstly, the utilization of the volume-translated Peng-Robinson (PR) EOS (Abudour et al. 2013) in this study is motivated by the reliance of the MMC model on accurate volume predictions within each cell. Employing the volume-translated PR-EOS can achieve more precise volume calculations for the fluids. Secondly, to eliminate the issue of numerical dispersion, the required total cell number is optimized to obtain a stable final recovery rate. Finally, the quadratic polynomial model estimates the recovery rate of infinite cells. Five oil-gas systems from the literature are used to validate the revised model’s effectiveness. Furthermore, the model illustrates the gas injection miscibility process, which helps us understand the miscibility mechanism.
Proposed MMP calculation algorithm
In the MMC model, the filled slim tube can be divided into many of the same volume cells. The process of continuously injecting gas is separated into batches, each possessing the same volume (as shown in Fig. 1). The MMC model has its foundation on four assumptions, as Yang et al. (2020) outlined. The assumptions include the following: (1) maintaining equal temperature and pressure throughout all cells; (2) no physical diffusion between cells; (3) no capillary forces within cells; (4) ensuring complete mixing within each cell. The present study involves conducting a thermodynamic P/T flash calculation in each cell, utilizing the volume-translated PR-EOS based on the given assumptions.
The MMC model’s schematic illustration (Yang et al. 2020)
The following is the comprehensive procedure for implementing the proposed MMC method of calculating MMP:
-
(1)
First, define the total number of cells (Nc=1000), the cell volume (Vc=1.0 cm3), the gas/oil ratio (GOR = 0.3), the initial pressure lower than MMP, the reservoir temperature, and the gas and oil composition. Each cell is initially filled using the same amount of original oil.
-
(2)
The total batch number of gas injections (Nb) is determined using the following equation:
According to Metcalfe et al. (Metcalfe et al. 1973), the required quantity of injected gas is 1.2 times the pore volume (PV) of the slim tube, which is also a well-known standard.
-
(3)
A volume of gas in each batch, GOR×Vc, is injected into the first cell. Then, a P/T flash calculation is conducted to calculate the volumes of the gas and liquid phases in the cell. After mixing the injected gas with the fluid in the cell, the equilibrium volume is greater than the cell volume. The excess volume of the equilibrium mixture over the cell volume is moved to cell 2. Figure 2 illustrates the detailed process of transferring the excessive volume. As shown in Fig. 2, if the equilibrium mixture is in the liquid phase, the excessive volume is transferred directly (scenario 1); if the equilibrium mixture is two phases and the volume of liquid phase is greater than the volume of cell, all gas phase, as well as a portion of the liquid phase, are transferred (scenario 2); if the mixture in equilibrium is two phases and the volume of liquid phase is less than the volume of cell, the gas phase that exceeds the cell volume is transferred (scenario 3).
-
(4)
The extra volume in the first cell is transferred to the second cell, and so on, until the last cell can produce oil.
-
(5)
When one batch computation is finished, a new batch begins in the first cell, and the cell-to-cell simulations, i.e., steps (3) and (4), are redone.
-
(6)
The following equation is utilized for estimating the oil recovery rate:
where Vo and Vr are the overall volume of initial oil in the slim tube and the amount of fluid recovered from cell Nc after 1.2 times PV of injected gas at atmospheric pressure and 298 K, respectively. The algorithm flowchart is demonstrated in Fig. 3.
Results and discussion
Summaries of studied oil-gas systems
This study has chosen five oil-gas systems (cases 1, 2, 3, 4, and 5) from the literature. The first three oil-gas systems consist of synthetic oil samples with three components driven by pure CO2 and various gas combinations as injected gas (as shown in Table 1). The PR-EOS properties and binary interactive parameters used in this study can be found in the literature (Jessen et al. 2004; Ahamadi 2011a; Yang et al. 2020).
Case 4
is a synthetic six-component oil (CO2 = 5%, CH4 = 20%, n-C4 = 5%, n-C10 = 40%, n-C14 = 10%, and n-C20 = 20%) driven by pure CO2 at a temperature of 71.1 °C (Wang et al. 1997; Zhao et al. 2006). Table 2 shows the oil and gas compositions and physical properties of the final oil-gas system (case 5), which consists of an actual live petroleum sample driven by pure CO2. The temperature for case 5 is 100 °C.
Improvements in recovery rate estimation
This work has used two cases (case 1 and case 2) to improve the recovery rate estimation. The binary interaction parameter (kij) of the PR-EOS and properties of the components are shown in the literature (Wang et al. 1997; Yang et al. 2007; Yang et al. 2020).
Optimizing total cell number
The Nc and GOR mainly influence the numerical dispersion. The smaller GOR not only increases the calculation accuracy of the oil recovery rate but also requires a smaller Nc to obtain a constant oil recovery rate (Zhao et al. 2006). In this study, The value of GOR is defined to be 0.3, as recommended by (Jaubert et al. 1998a and 1998b). However, Nc is not widely accepted as a criterion for eliminating numerical dispersion. An appropriate choice of Nc ensures a stable oil recovery rate. For case 1, the final recovery rate versus Nc is shown in Fig. 4.
As demonstrated in Fig. 4, the final oil recovery rate increases as the N c and pressure increase. The final oil recovery rate for 9 MPa becomes stable at the Nc of 100. However, the final oil recovery rate for 11.3 MPa is constant at the Nc of 500. Therefore, the Nc of 500 is required in all MMP calculations.
Fitting model
A modified model calculates the oil recovery rates at different Nc at a given pressure. These oil recovery rates determine the infinite-cell oil recovery factor (RF1.2∞). In the Jaubert algorithm (Jaubert et al. 1998a), RF1.2∞is obtained by extrapolating the linear relationship between RF1.2 and \(1/\sqrt{{N}_{\text{c}}}\). In this study, we use the second-order polynomial model to improve the estimation of RF1.2∞.Two different scenarios have been chosen for evaluating the fitting effect of two models. The first scenario is case 1 at 11 MPa; another is case 2 at 29 MPa. In each scenario, nine oil recovery rates are calculated at different Nc. The first four oil recovery rates (from the 100, 200, 300, and 400 cell simulations) are applied to fit each curve.
In comparison, the fitting is evaluated using the latest five oil recovery rates (calculated from the 500, 600, 700, 800, and 900 cell simulations). Figure 5 depicts the two scenarios. The fitted linear and second-order polynomial models in scenario 1 of Fig. 5 are similar, and both accurately determine the latest five recovery rates. In scenario 2 of Fig. 5, it is clear that the fitted second-order polynomial model estimates the recovery factor better, which is marked by a circular shape. The second-order polynomial equation better matches the fitted curves. Therefore, the new fitted model provides a more accurate prediction of RF1.2∞.
Validation of the modified method
In order to validate the estimation accuracy of the modified MMC method, the modified method is used to predict the MMP for five cases (cases 1, 2, 3, 4, and 5). Initially, a selection is made of three or four suitable pressures, which lie within the range of the bubble point’s pressure and the first contact miscibility pressure. The requirements for selecting pressure are determined by the oil recovery factor in the infinite-cell model, which ranges from 10 to 95%. A second-order polynomial model calculates the corresponding RF1.2∞ at the selected pressures. Afterward, these RF1.2∞s are used to estimate the MMP. Figure 6. demonstrates the exponential relationship between RF1.2∞ and pressure for cases 3 and 5. R2 denotes the value of the correlation coefficient, and the equation representing a straight line can be expressed as RF1.2∞ =0.97. P = MMP is the formula of the dashed line. This MMP is determined by the intersection points of the exponential curve and the straight line equation (RF1.2∞ =0.97).
Table 3 shows the comparison of MMP values from this study with the literature and their relative errors. The MMP values from this study are in better agreement with the results of the slim tube test, the MMC, and the MOC, as demonstrated in Table 1. The average relative error is less than 3.96%. The results suggest that the improved approach to MMP calculation exhibits high accuracy.
Visualization of miscibility process
To visualize the displacing front, a thermodynamic parameter is recorded as a function of time in each cell. As the simulation progresses, the total amount of gas added into the first cell calculates time. In the slim tube test, time is expressed by the PV of the gas injected. In this study, the critical distance, dc, is introduced (Jaubert et al. 1998b):
where ρL and ρV are the liquid and gas phase densities, respectively. When a mixing cell approaches the critical point, the gas-liquid density is the same, i.e., dc=0. The fluid within a cell approaches a critical point when its critical distance approaches zero. For Case 1, at a given pressure (close to MMP), the dc versus time curves in the 100th mixing cell are shown in Fig. 7.
As seen in Fig. 7, the oil tie-line appears first in the mixing cell. As time increases, the two-phase fluid eventually reaches the injected gas tie-line (as illustrated on the right side of Fig. 6). Furthermore, the condensation front (dc is decreasing) and the evaporation front (dc is increasing) connect the oil tie-line and the injection gas tie-line. The crossover tie-line is always found between two adjacent fronts. The crossover tie-line becomes smaller as the pressure increases. The crossover tie-line governs the miscibility process since it is closer to the critical point, corresponding to the crossover tie-line minimum value of dc in Fig. 7. The crossover tie-line divides the curve into condensing and vaporization zones. The oil’s intermediate components evaporate into the gas phase at the vaporization front, which is subsequently replaced by the injection of new gas, and the intermediate-rich gas phase is delivered to the next mixing cell. As time passes in a mixing cell, the oil phase becomes heavier, and the gas phase becomes lighter. Evaporation exacerbates the component differences in the mixing cell between the liquid and newly injected gas phases. As a result, the vaporizing front exhibits an increase in dc. The gas’s intermediate components condense into oil at the condensation front. They are subsequently replaced by the freshly injected rich gas, with the lighter gas phase transferred to the next mixing cell. For a given mixing cell, as time increases, there is an increasing number of intermediate components in the oil and gas phases, thus minimizing the difference between the liquid and gas phases’ components. A decrease in dc, therefore, characterizes the condensing front. As observed in Fig. 7, as increases in pressure, the minimum critical distance decreases, and the transition zone narrows and approaches the miscibility.
To better understand the variation of parameters such as gas-oil density, oil saturation, and gas-oil components in the miscibility process, Fig. 8 shows the parameter change curves as time in the 100th mixing cell for Case 1 at a given pressure of 11MPa (close to MMP).
According to the characteristics of gas-oil density changes, the curve in Fig. 8a can be divided into three parts: the initial oil zone, the oil-gas miscibility transition zone, and the gas zone. The formation process of the CVD miscibility mechanism of Case 1 is shown in Fig. 8a. In the oil-gas miscibility transition zone, the oil-gas density has the closest point at time 0.15 PV. Bounded by this point, the gas-liquid density changes with a condensation miscibility front on the left side and an evaporation miscibility front on the right. As shown in Fig. 8b, after time 0.11 PV, the content of CH4, n-C4, and n-C10 in the liquid phase is lower than that of the initial oil, and the content of n-C10 in the gas phase has a small change, which indicates that the CH4 and n-C4 evaporate into the gas phase and are transported to the next mixing cell. The higher CO2 level than the initial oil suggests that the CO2 condenses into the oil. The CO2 condensation makes the oil lighter, and then the CH4 and n-C4 evaporate, making the oil heavier. This reason is that oil and gas miscibility occurs in the middle of the transition zone.
Conclusions
This study proposes a modified multiple-mixing-cell(MMC) approach based on an initial cell-to-cell calculation model. The result is as follows:
-
1.
To improve the accuracy of the oil recovery rate, a required total cell number of 500 can ensure a stable oil recovery rate, which can eliminate the numerical dispersion issue. The second-order polynomial equation provides a more accurate infinite-cell oil recovery factor prediction.
-
2.
The minimum miscibility pressure(MMP) values obtained with the modified MMC approach exhibit a higher level of concordance with the MMPs reported in the literature (MMC method, method of characteristics(MOC) model, and slim-tube test). The average relative error is less than 3.96%. The revised MMC method offers high precision in the estimation of the MMP.
-
3.
The critical distance versus time plots and profiles visualize the simultaneous process of slim-tube experiments, which reveal the features of mass transfer between gas and oil phases and the miscibility process from different perspectives. As the pressure increases, the critical distance in minimum value decreases, and the oil-gas miscibility’s transition zone becomes narrower and closer to miscibility.
-
4.
The modified MMC algorithm can quickly determine the miscibility mechanism and visualize the dynamic miscibility process of multiple oil and gas contacts in a slime tube.
Data availability
The data utilized to support the study’s findings are provided in the article.
Abbreviations
- dc :
-
Critical distance
- Nb :
-
The total batch number of gas injections
- Nc :
-
The total number of cells
- Pc :
-
Critical pressure, MPa
- RF1.2 :
-
Recovery factor after 1.2 pore volume of injected gas
- RF1.2 ∞ :
-
Infinite-cell oil recovery factor
- Tc :
-
Critical temperature, K
- Vc :
-
The cell volume, mL
- Vo :
-
The overall volume of initial oil in the slim tube at atmospheric pressure and 298 K, mL
- Vr :
-
The amount of fluid recovered from cell Nc after 1.2 times PV of injected gas at atmospheric pressure and 298 K, mL
- ρ:
-
Density, g/cm3
- ω :
-
Acentric factor
- CCUS:
-
Carbon capture, utilization, and storage
- CD:
-
Condensing displacement
- EOS:
-
Equations of state
- GOR:
-
The gas/oil ratio
- MCM:
-
Multiple contact method
- MMC:
-
Multiple-mixing-cell
- MMP:
-
Minimum miscibility pressure
- MOC:
-
Method of characteristics
- PR-EOS:
-
Peng-Robinson Equation of State
- PV:
-
Pore volume
- RBA:
-
Rising bubble apparatus
- VCD:
-
Vaporizing and condensing displacement
- VD:
-
Vaporizing displacement
- VIT:
-
Vanishing interfacial tension
References
Abudour A, Mohammad S, Robinson R et al (2013) Volume-translated Peng-Robinson equation of state for liquid densities of divers binary mixtures. Fluid Phase Equilib 349:37–55
Ahmadi K, Johns RT (2011b) Multiple mixing-cell method for MMP calculations. SPE J 16(4):733–742. https://doi.org/10.2118/116823-PA
Ahmadi K, Johns RT, Mogensen K et al (2011a) Limitations of current method-of- characteristics (MOC) methods using shock-jump approximations to predict MMPs forcomplex gas/oil displacements]. SPE J 16(4):743–750. https://doi.org/10.2118/129709-PA
Ali S, Masoud R (2022) Estimating the minimum miscibility pressure(MMP) of methane-live oil using the slime tube test by modified oil recovery factor(MORF) and break-over pressure(MBOP) criteria. Petrol Sci Technol 41(7):713–730. https://doi.org/10.1080/10916466.2022.2069817
Dindoruk B, Johns R, Orr Jr FM (2021) measurement and modeling of minimum miscibility pressure: a state-of-the art review. SPE Res Eval Eng, 24(2):367–389. https://doi.org/10.2118/200462-PA
Elsharkawy AM, Poettmann FH, Christiansen RL (1996) measuring minimum miscibility pressure: slim-tube or rising bubble method? Energy Fuels, 10(2):443–449. https://pubs.acs.org/doi/https://doi.org/10.1021/ef940212f
Ge D, Cheng H, Cai M et al (2021) (2021)a new predictive method for CO2-Oil minimum miscibility pressure. Geofluids 1–8. https://doi.org/10.1155/2021/8868592
He C, Mu L, Xu A et al (2019) Phase behavior and miscible mechanism in the displacement of crude oil with associated sour gas. Oil Gas Sci Technol-Rev IFP Energies Nouvelles 74:54. https://doi.org/10.2516/ogst/2019024
Hearn CL, Whitson CH (1995) Evaluating miscible and immiscible gas injection in the Safah field Oman. SPE 29115, SPER reservoir Simulation Symposium, San Antonio, Texas, USA. https://doi.org/10.2118/29115-MS
Jaubert J-N, Wolff L, Neau E et al (1998a) A very simple multiple mixing cell calculation to compute the Minimum Miscibility pressure whatever the displacement mechanism. Ind Eng Chem Res 37(12):4854–4859. https://doi.org/10.1021/ie980348r
Jaubert J-N, Arras L, Neau E et al (1998b) properly defining the classical vaporizing and condensing mechanisms when a gas is injected into a crude oil. Ind Eng Chem Res, 37 (12): 4860–4869 https://doi.org/10.1021/ie9803016
Jessen K, Stenby EH, Orr FM Jr. (2004) Interplay of Phase Behavior and Numerical Dispersion in Finite-Difference Compositional Simulation. SPE J 9(2):193–201. https://doi.org/10.2118/88362-PA
Li R, Li H (2019) A modified multiple-mixing-cell algorithm for minimum miscibility pressure p rediction with the consideration of asphaltene-precipitaiton effect. Ind Eng Chem Res 58(33):15332–15343
Li J, Wang H, Xiao Q et al (2022) Development status of global CO2 flooding and storage technology. J Chongqin Univ Sci Technol (Nat Sci Ed) 24(4):103–108
Liao C, Liao X, Chen J et al (2014) Correlations of minimum miscibility pressure for pure and impure CO2 in low permeability oil reservoir. J Energy Inst 87(3):208–214. https://doi.org/10.1016/j.joei.2014.03.012
Metcalfe RS, Fussell DD, Shelton JL (1973) A multicell equilibrium separation model for the study of multiple contact miscibility in rich-gas drives. SPE J 13(3):147–155. https://doi.org/10.2118/3995-PA
Moghaddam AK, Dehaghani AHS (2017) Modeling of asphaltene precipitation in calculation of minimum miscibility pressure. Ind Eng Chem Res 56:7375–7383
Mutailipu M, Jiang L, Liu X et al (2019) CO2 and alkane minimum miscible pressure estimation by the extrapolation of interfacial tension. Fluid Phase Equilibria 494:103–114. https://doi.org/10.1016/j.fluid.2019.05.002
Orr FM Jr, Jessen K (2007) An analysis of the vanishing interfacial tension technique for determination of minimum miscibility pressure. Fluid Phase Equilibria 255(2):99–109. https://doi.org/10.1016/j.fluid.2007.04.002
Shojaei H, Rastegar R, Jessen K (2012) Experimental and modeling study of multicontact miscible displacements. SPE Improved Oil Recovery Symposium,Tulsa, Oklahoma, USA. https://doi.org/10.2118/154307-MS
Stalkup FI (1990) Effect of gas enrichment and numerical dispersion on enriched-gas-drive prediction. SPE Res Eng 5(4):647–655. https://doi.org/10.2118/18060-PA
Sun Y, Zuo L, Li X et al (2023) Enhancing shale gas recovery by carbon dioxide injection: a method of carbon capture, utilization and storage(CCUS). Process Saf Environ Prot 179:484–492. https://doi.org/10.1016/j.psep.2023.09.049
Wang Y, Orr FM (1997) Analytical calculation of Minimum Miscibility pressure. Fluid Phase Equilibr 139(1–2):101–124. https://doi.org/10.1016/S0378-3812(97)00179-9
Yang F, Zhao G, Adidharma H et al (2007) Effect of oxygen on minimum miscibility pressure in carbon dioxide flooding. Ind Eng Chem Res 46(4):1396–1401. https://doi.org/10.1021/ie061279g
Yang F, Yu P, Zhang X (2020) Multiple-mixing-cell model for calculation of minimum miscibility pressure controlled by tie-line length. Geofluids 2020(9587254). https://doi.org/10.1155/2020/9587254
Zhang K, Jia N, Zeng F et al (2019) A review of experimental methods for determining the oil- gas minimum miscibility pressure. J Petrol Sci Eng 183:1–24. https://doi.org/10.1016/j.petrol.2019.106366
Zhao G, Yang F, Towler B et al (2006) A New Approach for Calculation of Minimum Miscibility Pressure Based on a Multiple-Mixing-Cell Model. AIChE Annual Meeting, San Francisco, CA, USA
Acknowledgements
The National Natural Science Foundation of China (Grant 22268001) and the Guangxi Provincial Natural Science Foundation, China (2018JJA120001) support the project financially.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there are no conflicts of interest regarding the publication of this paper.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Yang, F. A modified cell-to-cell simulation model to predict oil-gas minimum miscibility pressure. J Petrol Explor Prod Technol (2024). https://doi.org/10.1007/s13202-024-01839-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13202-024-01839-y