Abstract
The demand for fuel from unconventional sources is increasing all over the world, however, there are still special and strict regulations regarding the methods of enhanced oil recovery as well as the content of the oil produced, including the amount of sulfur. In-situ combustion (ISC) is an attractive thermal method to enhance oil recovery and in-situ upgrading process. In this work, copper (II) oleate and copper (II) stearate were used for the oxidation of extra heavy oil with high sulfur content in the ISC process using a self-designed porous medium thermo-effect cell (PMTEC) and visual combustion tube. Using PMTEC the catalytic performances of the synthesized oil-soluble copper (II) oleate and copper (II) stearate and kinetic parameters such as activation energy using Ozawa-Flynn-Wall method were studied. The X-ray diffraction (XRD) and high-resolution field emission scanning electron microscopy were used to examine the characteristics of in-situ synthesized CuO nanoparticles during oxidation. As shown, the presence of oil-soluble copper (II) stearate and copper (II) oleate reduced oil viscosity from 9964 to 8000 and 6090 mPa˙s, respectively. Following ISC process in porous media in the presence of copper (II) oleate, the high sulfur extra heavy oil upgraded, and its sulfur content decreased from 10.33 to 6.79%. Additionally, SARA analysis revealed that asphaltene and resin content decreased in the presence of oil-soluble catalysts. During the oxidation reaction, homogeneous catalyst decomposed into nanoparticles, and heterogeneous catalyst is distributed uniformly in porous media and played an active role in the catalytic process. It should be noticed that, these kind of oil-soluble catalysts can be novel and highly potential candidates for initiation and oxidation of extra heavy oil in order to decrease the viscosity, enhanced oil recovery and production of the upgraded oil.
Graphical abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In-Situ Combustion (ISC) is one of the thermal methods for recovery of heavy and extra heavy oil. The air enters the oil reservoirs, and chemical or electrical operations or other materials and catalysts lead to ignition and temperature rise. The combustion process is accompanied by a decrease in the viscosity of the oil and a decrease in the amount of sulfur and heavy compounds (Castanier and Brigham 2003; Kök and Gul 2013; Deniz-Paker and Cinar 2017; Ismail and Hascakir 2020; Zhao et al. 2023). In comparison to other oil recovery techniques such as cyclic steam stimulation (CSS) (Wang et al. 2018a) and steam-assisted gravity drainage (SAGD) (Wang et al. 2020), the ISC method has unique advantages, including low heat loss due to the combustion process that occurs in reservoirs as well as abundant air supply (Varfolomeev et al. 2022). This process generates a significant amount of heat. Through this heat, the viscosity of heavy oil is reduced, thus increasing its mobility toward the production well for recovery (Al-Saffar et al. 2000). With such a high percentage of failed projects in the 1960s and the low probability of ISCs being successful, it might be argued that ISCs are a very complex and currently not well-understood process. As long as it is designed properly for the right type of reservoir, this process can recover oil with a significant percentage of crude oil-in-place (Sarathi 1999).
Three distinct zones of chemical and physical reaction were found in the ISC process, including 1) low-temperature oxidation (LTO), T < 350 °C, 2) fuel deposition (FD), T\(\sim\)350–450 °C, and 3) high-temperature oxidation (HTO), T > 450 °C (Varfolomeev et al. 2016; Kök et al. 2018; Mehrabi-Kalajahi et al. 2018, 2022; Zhao et al. 2024). The crude oil is oxidized in the first region, forming hydroperoxides, alcohols, aldehydes, ketones, as well as methane, ethane, CO, and CO2, which are gaseous products (Babapour Golafshani et al. 2023; Mehrabi-Kalajahi et al. 2024). The second stage uses pyrolysis to decompose the obtained products of the previous process into coke, which is then provided a fuel in the third stage to ensure propagation through a well and stability of the combustion process (Sadikov et al. 2018; Yuan et al. 2019; Rojas et al. 2021). One of the main reactions taking place in the third stage is the combustion of coke.
Regarding the recovery of heavy and extra-heavy oil from reservoirs, ISC technology has been considered a suitable option due to its low cost and low energy requirement for increasing oil production (Ismail et al. 2018; Popov et al. 2021). Taking advantages of the ISC, this process still suffers from some points, such as the difficulty of initiating the combustion and the instability of the combustion front in porous media, which make it difficult to enhance oil recovery (Xu et al. 2018; Abishev et al. 2018; Yuan et al. 2022). A number of factors can be used to overcome these disadvantages, including improving the combustion process using oil-soluble catalysts (Rezaei et al. 2013; Galukhin et al. 2016; Sadegh Mazloom et al. 2020; Zhao et al. 2020; Babapour Golafshani et al. 2021; Mehrabi-Kalajahi et al. 2021a, 2022; Saifullin et al. 2022).
Recently, metal-based catalysts have attracted more attention because of their capability to oxidize and crack hydrocarbons in porous media (Lin et al. 2017; Wang et al. 2018b; Lei et al. 2020). It is noteworthy, though, that both LTO and HTO onsets can be reduced by applying metal-based catalysts, including metal oxides and water-soluble nanoparticles (Kok and Bagci 2004; Mehrabi-Kalajahi et al. 2021a; Zhao et al. 2021b; Yuan et al. 2021). Since these kinds of catalysts are insoluble, poorly distributed in crude oil environments, and don’t make direct contact with crude oil during oxidation, they don’t generate much interest. In light of these negative aspects of metals, metal oxides, and water-soluble metal nanoparticle catalysts and precursors, ISC technology has emphasized the need for other targeted species of catalysts and precursors which are widely available and more readily soluble in crude oils, a controversial issue that continues to persist (Ramírez-Garnica et al. 2008). In previous works from our group, it was shown that when oil-dispersed or oil-soluble metal-based catalysts with a high capacity state are used in crude oil environments in the ISC process, the stability of combustion fronts changes dramatically (Babapour Golafshani et al. 2021, 2023; Mehrabi-Kalajahi et al. 2021b). The use of oil-soluble and oil-dispersed catalysts in porous media has been contemplated recently due to their widespread distribution in crude oil and their potential to enhance oil recovery and reduce viscosity (Zhao et al. 2020, 2021a; Mehrabi-Kalajahi et al. 2021a). Previous research by our team has shown that iron oxide coated with oleic acid (Fe2O3@oleic acid) is widely distributed in heavy oil, including liquid oil and porous media. This leads to a decrease in the ignition temperature and oxidation temperature in both the LTO and HTO stages of heavy oil (Mehrabi-Kalajahi et al. 2021a). It is possible to obtain in-situ metal oxide nanoparticles and achieve uniform dispersion of particles in the presence of oil-soluble catalysts. For instance, NiO nanoparticles were generated in-situ and deposited in coke-like residue to increase the rate at which asphaltenes oxidize (Amrollahi Biyouki et al. 2018).
During upgrading and refining to provide transportation fuels and petrochemicals, heavy oil and extra heavy oil still requires desulfurization and viscosity reduction (Javadli and De Klerk 2012; Mozhdehei et al. 2019; Urazov and Sviridenko 2021). For in-situ upgrading of heavy oil and extra heavy oils through the ISC process, stable combustion fronts are required. When oil-soluble catalysts are present, the ISC process can provide a stable combustion front, enhancing the quality of the combustion processes and reducing the amount of fuel that is deposited. Additionally, a proper combustion process can reduce the sulfur concentration and, in turn, upgrade the extra-heavy oil. In the catalytic reaction, it has been suggested that desulfurization of the heavy fractions contributes most to the reduction of viscosity of heavy oil. Several transition metal catalysts have been reported to create strong interaction with heavy oil structures and act as catalytic centers in the reaction (Rosales et al. 2006; Li et al. 2013). The result is that some large hydrocarbon molecules become partially unstable, which results in their decomposition into smaller oil components (Zhao et al. 2021a).
The purpose of this study is to investigate the possibility of upgrading extra heavy oil by reducing its viscosity and desulfurization in the presence of catalysts dispersed in oil with in-situ combustion process. To simulate and investigate the oxidation of extra heavy oil in porous media under airflow rate and displacement processes, a porous medium thermo-effect cell (PMTEC) and visual combustion tube (VCT) have been developed, which are accessible and not expensive. Aiming to improve the quality of extra heavy oil and decrease its viscosity and sulfur during the oxidation process, we investigated the effect of two different oil-soluble transition metal-bearing catalysts. Certain catalysts contain organic ligands in their structure that make them oil-soluble, distinguishing them from other metal-based catalysts (Amanam and Kovscek 2017; Amrollahi Biyouki et al. 2018; Yuan et al. 2021). This property enables better distribution in extra-heavy oil, making them highly promising for accelerating the oxidation process of this type of oil. The organic ligands help lower activation energy parameters and promote the production of coke, further enhancing their potential.
Experimental
Materials
An extra heavy oil sample was selected in this study. Table 1 displays the extra heavy oil’s physical and chemical characteristics. TatChemProduct provided the silica gel (Acros 24,037), (Kazan, Russia). Based on the ASTM D 4124 standard, the SARA analysis of extra heavy oil was carried out. Using a PerkinElmer 2400 Series (II) analyzer in accordance with ASTM (P/N 0240-1289), the initial extra heavy oil and upgraded oils’ elemental composition (C, H, N, and S) were analyzed (Varfolomeev et al. 2015).
Preparation of copper (II) stearate and copper (II) oleate
The analogous procedure we employed in our earlier researches (Sadikov et al. 2018; Yuan et al. 2019) was used to synthesize the copper (II) stearate and copper (II) oleate in the lab. For this purpose, we have used the saponification reaction method for synthesizing oil-soluble copper-based catalysts in the mixed solvent of water and ethanol in the presence of oleic acid, stearic acid, and copper salt (1 & 2).
After the extraction, the product was filtered and repeatedly washed with distilled water and ethanol (1:1) mixtures.
Extra heavy oil oxidation experiments
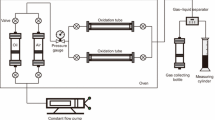
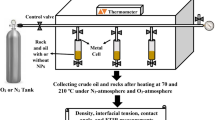
The oxidation of extra heavy oil was studied using the self-designed porous medium thermo-effect cell (PMTEC) and visual combustion tube (VCT), both with and without oil-soluble catalysts. The temperature variations occurring during the combustion reactions were observed using these well-designed equipment. The scheme of a PMTEC is shown in Fig. 1. To measure external temperatures (T1) and sample temperatures (T2), thermocouples were placed inside silica gel particles prior to and inside the oil samples. The ceramic heater had the following heating schedule. Following a 10 min thermal hold at 50 °C for the ambient temperature, the heater began to heat up to 700 °C at a rate of 10 °C/min. The extra heavy oil and silica gel were mixed at a ratio of 1 to 2 to prepare the sample for the PMTEC studies, and 1.5 g of the combined mass was added to the system. 1% was chosen as the catalyst’s optimum concentration for the extra heavy oil oxidation based on our prior study. Air was fed into the reactor at a rate of 0.2 l/min throughout the reactions. The more comprehensive details on PMTEC have been disclosed in our earlier works (Yuan et al. 2019, 2020). The self-designed Visual Combustion Tube (VCT) is used to study the oxidation of extra heavy oils in porous media at laboratory scale and under specific airflow conditions. The movement of the combustion front, the recovery rate of the recovered oil, and an examination of its characteristics using the SARA technique are the oxidation parameters in porous media that this equipment can provide. The sample (extra heavy oil + silica gel) was placed within a 1.5 cm-diameter, transparent quartz tube to prevent further interaction between the sample and the tube wall. For the purpose of tracking the movement of the combustion front during the oxidation, a thermometer was placed inside the tube at a fixed distance from the entrance of the sample. To provide the high temperature conditions for the start of the oxidation reaction in the sample, an electrical heater was attached around the quartz tube. Extra heavy oil and porous media (silica gel) have a ratio of 1 to 3, respectively. Within the oxidation reaction, the airflow was adjusted to 0.25 l/min. By using the viscosity measurement and SARA method, the recovered upgraded oils were examined individually (Mehrabi-Kalajahi et al. 2022).
Kinetics calculation extra of heavy oil combustion
Kinetic analysis of the crude oil oxidation has been widely measured using isoconversional methods. In this work, for the analysis of the activation energy (Eα), we have used Ozawa-Flynn-Wall (OFW) as the isoconversional method. It is possible to categorize regarding to the integral mathematical manipulations of the equations of Arrhenius (Vyazovkin et al. 2014; Yuan et al. 2021) (3):
where (A) constant pre-exponential factor (or simply the pre-factor) has been provided and it has units of s−1, R is the universal gas constant (8.314 J/mol K), Eα is the activation energy of the reaction (kJ/mol), and f(α) that describes the reaction mechanism for multi-step kinetics. Therefore the presented equation is used as the OFW method (4):
where β is the linear heating rate (in the unit of K/min), g(α) is the integral expression of kinetic model function, and Tα is temperature at conversion α. The activation energy (Eα) has been estimated by plotting ln(β) versus 1/T (K−1) at conversion α.
Characterization of obtained solid products using XRD and FESEM
The X-ray diffraction (XRD) technique was employed for the study of extra heavy oil + catalyst following isothermal treatment at high temperatures. It was conducted using a desktop diffractometer, model number MD-10 (Rodicon, St. Petersburg, Russia), outfitted with a position-sensitive detector and a Fe-K radiation source (with a wavelength of 1.93728 nm). In terms of angular accuracy for the position of the reflex, the diffractometer operates in a Debye-Scherer geometry and has a tolerance of roughly 0.02°. At room temperature, XRD patterns with a step of 0.015° were captured in the range of 17–130°. The characterization of the generated particles during the oxidation process was carried up in this investigation using a high-resolution field emission scanning electron microscopy (FESEM) fitted with an energy dispersive X-ray (EDX) analysis equipment (Aztec, Oxford Instruments) (Yu et al. 2011).
Results and discussion
The combustion process of extra heavy oil under airflow in PMTEC
We investigated the properties of extra heavy oil and the effect of catalysts on oxidation reactions using PMTEC. In these experiments, the synthesized catalysts were introduced to the extra heavy oil environment and porous media using solvent (CH2Cl2) and mechanically mixed very well in order to obtained homogeneous sample. It should be noticed that, due to the boiling point of the dichloromethane, during the mixing process it will evaporated. The PMTEC experiments recorded three different temperature curves: (1) the environment temperature (T1), (2) the sample temperature (T2), and (3) the temperature difference between the environment and the sample (T2T1). T1, which illustrates the heating process of the inert atmosphere, contains no samples. T2 displays the temperature of the oil sample in the porous medium based on the interactions of the extra heavy oil with the injected air during the heating process. The difference between T1 and T2, which results from both chemical and physical reactions occurring in the porous medium, equals the net increase in temperature of these two dissimilar contents. The temperature profiles of extra heavy oil acquired from PMTEC are shown in Fig. 2. The fact that the initial ambient temperature (T1) and sample temperature (T2) were similar demonstrates the porous media’s ability to maintain a constant temperature. The oxidation began in the reactor and produced the temperature differential over time when the heat treatment process was increased. In effect of the small amount evaporation of light fractions of oil and small amount of moisture absorbed by silica gel, there was a negative temperature differential between 85 and 130 °C. Interesting combustion temperature peaks were seen in the sample temperature (T2) curve in the ranges of 221–295 °C. Notably, the temperature profile produced from PMTEC shows the shoulder before to the oxidation peak temperature. This shoulder can be attributed to the initial phases of oxidation and the exothermic action of oxygen addition activities (Pu et al. 2019).
The Fig. 3 and Table 2 illustrate the effect of copper (II) oleate and copper (II) stearatre on extra heavy oil oxidation when combined with a dynamic 0.2 l/min airflow. Copper (II) oleate and copper (II) stearatre demonstrate that their presence has significant effect on the oxidation intervals. The PMTEC experimentation’s data here are dissimilar from those of the differential scanning calorimetry experiment (DSC). For instance, the peak that was produced in these experiments is referred to as low-temperature combustion process (LTC), as opposed to low-temperature oxidation (LTO), which was produced in DSC experiments. The LTC was seen to be pushed into the lower temperatures in the presence of oil-soluble copper (II) oleate and copper (II) stearatre as catalysts. As it is seen, with catalysts, the temperature range of the combustion process declined from 221–295 to 213–284 and 219–285 °C, respectively. Additionally, the peak combustion temperatures dropped from 240 to 233 and 236 °C. The presence of oleic acid and stearic acid as organic ligands during the pre-combustion stage can also promote the oxidation reactions since they contain carboxylic acid as the favorable and promising product. In comparison to other copper-based research, the inclusion of organic ligands in the catalysts’ structure enhanced the dispersion of oil-soluble catalysts in extra heavy oil. In this study, the on-set temperature of LTC decreased from 239 to 213 °C with the presence of copper (II) oleate compared to CuO nanoparticles. (Amanam and Kovscek 2017). Compared to other studies, the use of in-situ synthesized nanoparticles as the active form of catalyst has been found to be more effective than the ex-situ synthesizing process in terms of improving the oxidation performance and reducing the viscosity of the produced oil (Amrollahi Biyouki et al. 2018). This is due to the fact that the in-situ process allows for better control over the size, shape, and distribution of the nanoparticles, resulting in an increased surface area and more efficient catalytic activity. Additionally, the in-situ method minimizes the agglomeration of nanoparticles, leading to a more uniform dispersion throughout the oil matrix and improved catalytic performance. These factors contribute to the superior performance of the in-situ synthesized nanoparticles as an active catalyst.
Impact of catalysts on the apparent activation energy of extra heavy oil oxidation reaction in the porous medium
To understand the effect of oil-soluble catalysts on the kinetics parameters of extra heavy oil oxidation, Eα was valued by OFW method. According to oxygen consumption during oxidation process at different heating rate the kinetic parameters were calculated. Figure 4 displayed the dependence of activation energy on conversion degree. It can be seen that due to oil-solubility of catalysts especially copper (II) oleate at the beginning of the oxidation reaction the value of activation energy is lower in the presence of catalysts, which shows in low temperate regions the effect of copper (II) oleate is higher than copper (II) stearate. It can be attributed to oxygen addition reaction that oleate can accelerated the oxidation reaction even in LTO stage. As can be seen from Fig. 4 with the increasing of conversion degree (α) both values of Eα with copper (II) oleate and copper (II) stearate are lower than without catalyst. The similarity of these catalysts in higher α can be related to formation of the same active from of catalysts (CuO) in high temperate regions. The results showed that the application of oil-soluble catalysts have good enough catalytic effect on the oxidation of extra heavy oil in porous media. The lower activation energy vales support the idea that the oxidation reaction in the presence of oil-soluble catalysts occur easier in a lower temperate.
Catalysts effect on extra heavy oil properties during oxidation in porous medium using VCT
Figure 5 displays the thermocouple-recorded combustion front propagation and temperature outline of extra heavy oil oxidation in porous medium. The porous media’s fixed thermocouple provided combustion front spreading information and monitored the coke combustion’s heat effect. The inclusion of oil-soluble catalysts accelerated the spread of the combustion front relative to the absence of catalyst conditions, as indicated by the temperature profile observed by the thermocouple. The combustion front’s spread was promoted more effectively by copper (II) oleate than by copper (II) stearate. To explore the chemical changes occurring during the oxidation process in the structure of the crude oil, the produced oils following the ISC process were evaluated by viscosity measurments and SARA method. According to Table 3, the viscosity reduced from 9964 to 8000 and 6090 mPa‧s in the presence of copper (II) stearate and copper (II) oleate, respectively. Based on the SARA analysis, the asphaltene and resin contents in recovered oils decreased from 23.1, 23.4 to 8.1, 10.2 and 7.6, 8.4% respectively, with the addition of the oil-soluble copper (II) stearate and copper (II) oleate. According to Table 3, when copper (II) stearate and copper (II) oleate were present, the amount of the saturate fractions after ISC improved from 20.6 to 42.9 and 46.1%, respectively. According to the oxidation results of using copper (II) stearate and copper (II) oleate as oil-soluble catalysts in this work and CoFe2O4@oleic acid, Fe2O3@oleic acid, TiO2@oleic acid, α-Fe2O3 and γ-Fe2O3 as oil-dispersed catalysts in previous works (Golafshani et al. 2019; Mehrabi-Kalajahi et al. 2021a, b; Yuan et al. 2021), it can be said that the use of oil-soluble copper (II) oleate, due to its high dispersion in ultra-heavy oil, produced a greater effect in reducing the oxidation temperature and promoting the ultra-heavy oil process. Gas chromatography (GC) was used to examine the carbon number variations in the achieved saturated fractions. As demonstrated in Fig. 6, the presence of catalysts, particularly copper (II) oleate, led to a drop in weightier alkanes and a rise in lighter alkanes. This may be connected to the process of in-situ upgrading of extra heavy oil and catalytic cracking of heavyweight components during the oxidation process.
Contaminants and high molecular weight hydrocarbons including nitrogen, sulfur, aromatics, asphaltenes, and heavy metals make up the majority of extra heavy oils. Sulfur is regarded as the most dangerous unwanted contaminant among these substances (Houda et al. 2018). Desulfurization is one of the most significant steps in upgrading oil in a refinery. Oxidation can reduce nitrogen and sulfur concentrations in crude oils through the transformation of these elements into acids (Kansha et al. 2022). It has been developed a number of desulfurization processes, including atmospheric distillation residue desulfurization as well as hydrogen desulfurization (Gary et al. 2007). The development of alternative nonhydrogenation processes has made significant progress. Desulfurization by adsorption and oxidative desulfurization are some of the processes for producing ultra-low sulfur fuel. Due to its characteristic towards high oxidative reactivity and sulfur molecules, oxidative desulfurization with an oxidizing agent has received significant attention (Houda et al. 2018). Herein, the elemental compositions of initial extra heavy oil and the produced oils after in-situ combustion with and without oil-soluble catalysts were listed in Table 3. In the produced oils, carbon, hydrogen, and the H/C ratio all increased with ISC and ISC with oil-soluble catalysts. In contrast, nitrogen and especially sulfur decreased with ISC and ISC with catalysts. The application of oil-soluble copper (II) stearate and copper (II) oleate in the ISC improved desulfurization and eliminated more sulfur from extra heavy oil. The elemental analysis experiments showed that, in the presence of copper (II) stearate and copper (II) oleate, respectively, the sulfur content decreased from the initial extra heavy oil’s 10.33 to 7.28 and 6.79%. In addition, the inclusion of copper (II) oleate, as an effective catalyst, enhanced the recovery factor and production of upgraded oil during the oxidation process of crude oil in porous medium from 44.7 to 52.6%.
Examination of in-situ transformation of catalyst within the oxidation of extra heavy oil
To better understand of catalytic oxidation mechanisms and in-situ transformation of oil-soluble copper-based catalysts in oxidation reactions, the isothermal oxidation experiments of extra heavy oil + copper (II) oleate in porous media carried out based on two combustion zone obtained from VCT at 500 and 800 °C. The samples’ XRD patterns at 500 and 800 °C are shown in Fig. 7A. The copper oxide is synthesized in-situ at higher temperatures (800 °C) as a result of the decomposition of CuSO4, which is interestingly detected at 500 °C due to the high sulfur concentration in the extra heavy oil structure. Reflexes associated with CuO are seen to be slightly stretched, which is consistent with their modest size as copper oxide nanoparticles (Babapour Golafshani et al. 2021). The SEM images of solid products produced by isothermal oxidation are shown in Fig. 7B. It is obvious that the acquired product morphologies at 500 and 800 °C differ from each other. The agglomerated and adhered particles are visible in the SEM image at 500 °C, but at higher temperatures, these accumulated particles were disseminated into the sample’s residual bulks and produced morphologies with greater porosities than at lower temperatures. Overall, it is clear from XRD and SEM that the in-situ synthesize of copper oxides is crucial to the process of oxidizing extra heavy oil, especially at high temperature oxidation (HTO). Earlier, it was established that the primary factor affecting the catalytic oxidation reaction of the heavy oil’s asphaltene is the dispersion of the nanoparticles inside of coke residue (Hosseinpour et al. 2014).
Conclusions
In this study, the copper (II) stearate and copper (II) oleate as oil-soluble catalysts were synthesized and studied in the ISC process of extra heavy oil for in-situ upgrading process and enhancing oil recovery. The PMTEC and VCT were applied to investigation of both oil-soluble catalysts in porous media for extra heavy oil oxidation. The following are the main results of the current study:
-
1.
Catalysts shifted the combustion temperature of extra heavy oil to lower temperatures, which in the presence of copper (II) oleate decreased from 240 to 233 °C.
-
2.
Kinetic calculations showed that the average activation energy decreased from 90 to 70 kJ/mol in the presence of copper (II) oleate as the superior catalyst.
-
3.
The combustion experiments using VCT showed that the applying copper-based catalysts accelerated the oil displacement in porous media. Furthermore, after ISC process with the addition of catalysts, the produced oil’s viscosity and the content of sulfur decreased from 9964 mPa˙s and 10.33% to 6090 mPa˙s and 6.79% in the presence of copper (II) oleate.
-
4.
The presence of oil-soluble copper (II) oleate reduced the heavy fractions share (resin + asphaltene) from 46.5 to 16%, and increased the lighter fractions share (saturate + aromatic) from 53.5 to 84%.
-
5.
Results from XRD and SEM confirmed that during the oxidation process, the organic parts of oil-soluble catalyst decomposed and the CuO nanoparticles in-situ formed.
-
6.
Low-cost and manageable precursors can be used to manufacture these types of catalysts. These novel catalysts compared to others metal-based catalysts have high potential for the ISC process of extra heavy oil, which can enhance oil recovery and in-situ upgrading process, as they possess unique characteristics compared to other metal-based catalysts.
Abbreviations
- CSS:
-
Cyclic steam stimulation
- EOR:
-
Enhanced oil recovery
- FD:
-
Fuel deposition
- FESEM:
-
High-resolution field emission scanning electron microscopy
- GC:
-
Gas chromatography
- HTO:
-
High-temperature oxidation
- ISC:
-
In-situ combustion
- LTO:
-
Low-temperature oxidation
- OFW:
-
Ozawa-Flynn-Wall
- PMTEC:
-
Porous medium thermo-effect cell
- SAGD:
-
Steam-assisted gravity drainage
- SARA:
-
Saturate, Aromatic, Resin and Asphaltene
- VCT:
-
Visual combustion tube
- XRD:
-
X-ray diffraction
- A:
-
Constant pre-exponential factor (s−1)
- Eα :
-
Activation energy (kJ/mol)
- g(α):
-
The integral expression of kinetic model function
- R:
-
The universal gas constant (8.314 J/mol K)
- Tα :
-
The temperature at conversion α
- \(\beta \) :
-
The linear heating rate (K/min)
References
Abishev A, Tokarev V, Sagyndikov M (2018) Evaluation of in-situ combustion efficiency in karazhanbas oilfield, Western Kazakhstan. In: SPE Annual Caspian Technical Conference. https://doi.org/10.2118/192553-MS
Al-Saffar H, Price D, Soufi A, Hughes R (2000) Distinguishing between overlapping low temperature and high temperature oxidation data obtained from a pressurized flow reactor system using consolidated core material. Fuel 79:723–732. https://doi.org/10.1016/S0016-2361(99)00210-0
Amanam UU, Kovscek AR (2017) Analysis of the effects of copper nanoparticles on in-situ combustion of extra heavy-crude oil. J Pet Sci Eng 152:406–415. https://doi.org/10.1016/j.petrol.2017.02.018
AmrollahiBiyouki A, Hosseinpour N, Nassar NN (2018) Pyrolysis and oxidation of asphaltene-born coke-like residue formed onto in situ prepared NiO nanoparticles toward advanced in situ combustion enhanced oil recovery processes. Energy Fuels 32:5033–5044. https://doi.org/10.1021/acs.energyfuels.8b00638
BabapourGolafshani M, Varfolomeev MA, Mehrabi-Kalajahi S et al (2021) Oxidation of heavy oil using oil-dispersed transition metal acetylacetonate catalysts for enhanced oil recovery. Energy Fuels 35:20284–20299. https://doi.org/10.1021/acs.energyfuels.1c03434
BabapourGolafshani M, Mehrabi-Kalajahi S, Varfolomeev M et al (2023) Improving the performance of heavy oil oxidation using oil-soluble copper-based catalysts in in situ combustion for the in situ upgrading process. Energy Fuels 37:6705–6714. https://doi.org/10.1021/acs.energyfuels.3c00455
Castanier LM, Brigham WE (2003) Upgrading of crude oil via in situ combustion. J Pet Sci Eng 39:125–136. https://doi.org/10.1016/S0920-4105(03)00044-5
Deniz-Paker M, Cinar M (2017) Investigation of the combustion characteristics of Bati Raman oil with sand. J Pet Sci Eng 157:793–805. https://doi.org/10.1016/j.petrol.2017.08.006
Galukhin A, Khelkhal MA, Gerasimov A et al (2016) Mn-catalyzed oxidation of heavy oil in porous media: kinetics and some aspects of the mechanism. Energy Fuels 30:7731–7737. https://doi.org/10.1021/acs.energyfuels.6b01234
Gary JH, Handwerk JH, Kaiser MJ, Geddes D (2007) Petroleum refining: technology and economics, 5th edn. Pet Refin, China
Golafshani MB, Varfolomeev MA, Yuan C, Kalajahi SM (2019) Oxidation of heavy crude oil using oil-soluble titanium oxide in in-situ combustion process. In: International Multidisciplinary Scientific GeoConference Surveying Geology and Mining Ecology Management, SGEM, 19(12): 1039–1044. https://doi.org/10.5593/sgem2019/1.2/S06.132
Hosseinpour N, Mortazavi Y, Bahramian A et al (2014) Enhanced pyrolysis and oxidation of asphaltenes adsorbed onto transition metal oxides nanoparticles towards advanced in-situ combustion EOR processes by nanotechnology. Appl Catal A Gen 477:159–171
Houda S, Lancelot C, Blanchard P et al (2018) Oxidative desulfurization of heavy oils with high sulfur content: a review. Catal 8:344. https://doi.org/10.3390/catal8090344
Ismail NB, Siu J, Hascakir B (2018) Kinetics analysis validation for in-situ combustion by coupling experimental data with analytical methods. Proc—SPE Annu Tech Conf Exhib. https://doi.org/10.2118/191745-MS
Ismail NB, Hascakir B (2020) Impact of asphaltenes and clay interaction on in-situ combustion performance. Fuel 268:117358. https://doi.org/10.1016/j.fuel.2020.117358
Javadli R, De Klerk A (2012) Desulfurization of heavy oil-oxidative desulfurization (ODS) as potential upgrading pathway for oil sands derived bitumen. Energy Fuels 26:594–602. https://doi.org/10.1021/ef201448d
Kansha Y, Kato S, Tsuji K (2022) Analysis of reactions during the residue desulfurization of heavy oil based on a data-driven method. Comput Chem Eng 164:107901. https://doi.org/10.1016/j.compchemeng.2022.107901
Kök MV, Gul KG (2013) Combustion characteristics and kinetic analysis of Turkish crude oils and their SARA fractions by DSC. J Therm Anal Calorim 114:269–275. https://doi.org/10.1007/s10973-013-3256-3
Kök MV, Varfolomeev MA, Nurgaliev DK (2018) Isoconversional methods to determine the kinetics of crude oils -thermogravimetry approach. J Pet Sci Eng 167:480–485. https://doi.org/10.1016/j.petrol.2018.04.040
Kok MV, Bagci S (2004) Characterization and kinetics of light crude oil combustion in the presence of metallic salts. Energy Fuels 18:858–865. https://doi.org/10.1021/ef0301755
Lei Z, Hao S, Yang J, Dan X (2020) Study on solid waste pyrolysis coke catalyst for catalytic cracking of coal tar. Int J Hydrogen Energy 45:19280–19290. https://doi.org/10.1016/j.ijhydene.2020.05.075
Li J, Chen Y, Liu H et al (2013) Influences on the aquathermolysis of heavy oil catalyzed by two different catalytic ions: Cu2+ and Fe3+. Energy Fuels 27:2555–2562. https://doi.org/10.1021/ef400328s
Lin X, Nie Z, Zhang L et al (2017) Nitrogen-doped carbon nanotubes encapsulate cobalt nanoparticles as efficient catalysts for aerobic and solvent-free selective oxidation of hydrocarbons. Green Chem 19:2164–2173. https://doi.org/10.1039/c7gc00469a
Mehrabi-Kalajahi S, Varfolomeev M, Yuan C et al (2021a) Oil-dispersed α-Fe2O3 nanoparticles as a catalyst for improving heavy oil oxidation. Energy Fuels 35:10498–10511. https://doi.org/10.1021/acs.energyfuels.1c00657
Mehrabi-Kalajahi S, Varfolomeev MA, Yuan C et al (2021b) Improving heavy oil oxidation performance by oil-dispersed CoFe2O4 nanoparticles in In-situ combustion process for enhanced oil recovery. Fuel 285:119216. https://doi.org/10.1016/j.fuel.2020.119216
Mehrabi-Kalajahi S, Hadavimoghaddam F, Varfolomeev MA et al (2022) Effect of different water content and catalyst on the performance of heavy oil oxidation in porous media for in situ upgrading. Ind Eng Chem Res 61:9234–9248. https://doi.org/10.1021/acs.iecr.2c01007
Mehrabi-Kalajahi S, Varfolomeev MA, Sadikov KG et al (2024) The impact of the initiators on heavy oil oxidation in porous media during in situ combustion for in situ upgrading process. Energy Fuels. https://doi.org/10.1021/acs.energyfuels.3c05068
Mehrabi-Kalajahi S, Varfolomeev M, Yuan C, et al (2018) Using EPR technique for monitoring of isc processes and reservoirs temperature in enhanced oil recovery. Soc Pet Eng—SPE Russ Pet Technol Conf 2018, RPTC 2018. https://doi.org/10.2118/191548-18RPTC-MS
Mozhdehei A, Hosseinpour N, Bahramian A (2019) Dimethylcyclohexylamine switchable solvent interactions with asphaltenes toward viscosity reduction and in situ upgrading of heavy oils. Energy Fuels 33:8403–8412. https://doi.org/10.1021/acs.energyfuels.9b01956
Popov E, Askarova A, Mukhametdinova A et al (2021) Evaluation of the applicability of in-situ combustion in a heavy oil carbonate field with high initial oil saturation. J Pet Sci Eng 207:109146. https://doi.org/10.1016/j.petrol.2021.109146
Pu W, Gong X, Chen Y et al (2019) Oxidation kinetic evaluation of the low temperature oxidized products of Tahe heavy oil characterized by the distributed activation energy model. J Pet Sci Eng 181:106155. https://doi.org/10.1016/j.petrol.2019.06.019
Ramírez-Garnica MA, Hernández-Pérez JR, Cabrera-Reyes MC, et al (2008) Increase oil recovery of heavy oil in combustion tube using a new catalyst based on nickel ionic solution. In: SPE International Thermal Operations and Heavy Oil Symposium 2008 Oct 20 (pp. SPE-117713). https://doi.org/10.2118/117713-MS
Rezaei M, Schaffie M, Ranjbar M (2013) Thermocatalytic in situ combustion: influence of nanoparticles on crude oil pyrolysis and oxidation. Fuel 113:516–521. https://doi.org/10.1016/j.fuel.2013.05.062
Rojas AA, Yuan C, Emelianov DA, et al (2021) A 3-Step reaction model for numerical simulation of in-situ combustion. In SPE Russian Petroleum technology conference Oct 12 (p. D011S004R003). Society of petroleum engineers. https://doi.org/10.2118/206430-MS
Rosales S, Machín I, Sánchez M et al (2006) Theoretical modeling of molecular interactions of iron with asphaltenes from heavy crude oil. J Mol Catal A Chem 246:146–153. https://doi.org/10.1016/j.molcata.2005.10.027
SadeghMazloom M, Hemmati-Sarapardeh A, Husein MM et al (2020) Application of nanoparticles for asphaltenes adsorption and oxidation: a critical review of challenges and recent progress. Fuel. https://doi.org/10.1016/j.fuel.2020.117763
Sadikov K, Yuan C, Mehrabi-Kalajahi SS, et al (2018) A new, fast, and efficient method for evaluating the influence of catalysts on in-situ combustion process for heavy oil recovery. In: SPE international heavy oil conference and exhibition, p. D032S043R001. SPE, 2018. https://doi.org/10.2118/193758-MS
Saifullin ER, Mehrabi-Kalajahi S, Yuan C et al (2022) Catalytic combustion of heavy crude oil by oil-dispersed copper-based catalysts: Effect of different organic ligands. Fuel 316:123335. https://doi.org/10.1016/j.fuel.2022.123335
Sarathi PS (1999) In-Situ combustion handbook—principles and practices. National energy technology (United States). National petroleum technology office (NPTO) No. DOE/PC/91008–0374
Urazov KK, Sviridenko NN (2021) NiO based catalysts obtained “in-situ” for heavy crude oil upgrading: effect of NiO precursor on the catalytic cracking products composition. J Taiwan Inst Chem Eng 127:151–156. https://doi.org/10.1016/j.jtice.2021.07.044
Varfolomeev MA, Nagrimanov RN, Galukhin AV et al (2015) Contribution of thermal analysis and kinetics of Siberian and Tatarstan regions crude oils for in situ combustion process. J Therm Anal Calorim 122:1375–1384. https://doi.org/10.1007/s10973-015-4892-6
Varfolomeev MA, Galukhin A, Nurgaliev DK, Kok MV (2016) Thermal decomposition of Tatarstan Ashal’cha heavy crude oil and its SARA fractions. Fuel 186:122–127. https://doi.org/10.1016/j.fuel.2016.08.042
Varfolomeev MA, Yuan C, Bolotov AV et al (2022) Case study on the application of in-situ combustion for ultra-low permeability oil shale from natih b formation (Oman): synthetic oil generation and micro-scale pore structure changes. Soc Pet Eng. https://doi.org/10.2118/211011-MS
Vyazovkin S, Chrissafis K, Di Lorenzo ML et al (2014) ICTAC Kinetics Committee recommendations for collecting experimental thermal analysis data for kinetic computations. Thermochim Acta 590:1–23. https://doi.org/10.1016/j.tca.2014.05.036
Wang Y, Ren S, Zhang L et al (2018a) Numerical study of air assisted cyclic steam stimulation process for heavy oil reservoirs: recovery performance and energy efficiency analysis. Fuel 211:471–483. https://doi.org/10.1016/j.fuel.2017.09.079
Wang Y, Zeng H, Shi Y, Cai N (2018b) Methane partial oxidation in a two-layer porous media burner with Al2O3 pellets of different diameters. Fuel 217:45–50. https://doi.org/10.1016/j.fuel.2017.12.088
Wang D, Qiao C, Zhao Z et al (2020) Effect of charge density of reverse emulsion breaker on demulsification performance for steam-assisted gravity drainage (SAGD) emulsions under high temperature and high pressure. Energy Fuels 34:13893–13902. https://doi.org/10.1021/acs.energyfuels.0c02690
Xu Q, Long W, Jiang H et al (2018) Pore-scale modelling of the coupled thermal and reactive flow at the combustion front during crude oil in-situ combustion. Chem Eng J 350:776–790. https://doi.org/10.1016/j.cej.2018.04.114
Yu BY, Elbuken C, Ren CL, Huissoon JP (2011) Image processing and classification algorithm for yeast cell morphology in a microfluidic chip. J Biomed Opt 16:066008. https://doi.org/10.1117/1.3589100
Yuan C, Sadikov K, Varfolomeev M et al (2020) Low-temperature combustion behavior of crude oils in porous media under air flow condition for in-situ combustion (ISC) process. Fuel 259:116293. https://doi.org/10.1016/j.fuel.2019.116293
Yuan C, Rodionov N, Mehrabi-Kalajahi S et al (2021) Catalytic combustion of heavy oil using γ-Fe2O3 nanocatalyst in in-situ combustion process. J Pet Sci Eng 209:109819. https://doi.org/10.1016/j.petrol.2021.109819
Yuan C, Pu WF, Emelianov DA et al (2022) Catalytic oxidation of alkanes by organometallics in an in situ combustion process. Energy Fuels 36:10167–10176. https://doi.org/10.1021/acs.energyfuels.2c02121
Yuan C, Mehrabi-Kalajahi SS, Sadikov K, et al (2019) Potential of copper-based oil soluble catalyst for improving efficiency of in-situ combustion process: catalytic combustion, catalytic in-situ oil upgrading, and increased oil recovery. In: SPE Kuwait Oil & Gas Show and Conference. Society of Petroleum Engineers, Mishref, Kuwait, p 13. https://doi.org/10.2118/198038-MS
Zhao S, Pu W, Varfolomeev MA et al (2020) Low-temperature combustion characteristics of heavy oils by a self-designed porous medium thermo-effect cell. J Pet Sci Eng 195:107863. https://doi.org/10.1016/j.petrol.2020.107863
Zhao F, Liu Y, Lu N et al (2021a) A review on upgrading and viscosity reduction of heavy oil and bitumen by underground catalytic cracking. Energy Rep 7:4249–4272. https://doi.org/10.1016/j.egyr.2021.06.094
Zhao R, Heng M, Chen C et al (2021b) Catalytic effects of Al2O3 nano-particles on thermal cracking of heavy oil during in-situ combustion process. J Pet Sci Eng 205:108978. https://doi.org/10.1016/j.petrol.2021.108978
Zhao S, Pu W, Jiang Q et al (2023) Investigation into the key factors influencing the establishment and propagation of combustion front in ultra-deep high-temperature heavy oil reservoirs. Energy 283:129017. https://doi.org/10.1016/j.energy.2023.129017
Zhao S, Pu W, Li Y et al (2024) Oxidation behavior and kinetics of shale oil under different oxygen concentrations. Fuel 361:130677. https://doi.org/10.1016/j.fuel.2023.130677
Acknowledgements
This work was supported by the Ministry of Science and Higher Education of the Russian Federation under agreement No. 075-15-2022-299 within the framework of the development program for a world-class Research Center “Efficient development of the global liquid hydrocarbon reserves”.
Funding
This research received no external funding.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Nejad Zare, R., Mehrabi-Kalajahi, S., Varfolomeev, M.A. et al. Improving the in-situ upgrading of extra heavy oil using metal-based oil-soluble catalysts through oxidation process for enhanced oil recovery. J Petrol Explor Prod Technol (2024). https://doi.org/10.1007/s13202-024-01813-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13202-024-01813-8