Abstract
Geochemical and biomarker characteristics of representative crude oil samples from selected fields in southern Nigeria were evaluated to determine the organic matter input, origin of biological material, depositional environment, thermal maturity, and genetic relationship between the oils. Four crude oil samples were obtained from various oil producing fields from Delta, Bayelsa and Abia state in southern Nigeria and labeled Kwale (KW), Kolo creek (KLC), Owaza (OWA1 and OWA2). The crude oil samples were fractionated into saturates, aromatic hydrocarbons and polar compounds using column chromatography on silica gel thereafter, analyzed using Gas chromatography-mass spectrometry (GC–MS). The calculated ratios of normal alkanes, acyclic isoprenoids, carbon preference index (CPI), hopanes, and steranes showed the following results: Pr/Ph (0.34 to 0.89); C29/C27 (0.78 to 1.25); 20S/(20S + 20R)C29sterane (0.28 to 0.66); 22S/(22S + 22R)C32 homohopane (0.17 to 0.23); CPI (0.96 to 0.98); Ts/Ts + Tm (0.46 to 0.50); and sterane/hopane (0.16 to 0.87). The results obtained were used to correlate the crude oils with respect to depositional environment, thermal maturity, and organic matter source. The Pr/Ph ratios of KW and KLC were less than one, and the cross-plot of Pr/nC17 versus Ph/nC18 of KW and KLC suggested that the oils were deposited under anoxic environments, whereas OWA1 and OWA2 indicated oxic conditions with no biodegradation. From the calculated ratios of 22S (22S + 22R)C32 homohopane and CPI, the oils were mature and had entered the generating window. Sample OWA1 is the most mature, while KLC is the least mature. The calculated ratios also showed that the four oil samples were from a shale source rock with both terrestrial and marine inputs.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The hydrocarbon-producing region of Nigeria is the Niger Delta, which is located on West Africa's Atlantic coast and comprises roughly 75,000 km2 with a projected estimate of crude oil and natural gas volumes of 37 billion barrels and 256 trillion cubic feet. The area is predicted to be one of the most productive hydrocarbon-producing basins in the world (EIA 2015). The southern region is recognized for its wealth of hydrocarbon resources and is presently the only basin that houses commercial quantities of petroleum resources in Nigeria. It contains a siliciclastic fill with an average thickness of 9000–12,000 m and covers around 75,000 km2. The region's main operational petroleum system is the tertiary Niger Delta. The Niger Delta basin's geology comprises principal geologic formations such as Agbada, Akata, and Benin (Abrakasa et al. 2016). The region's depobelt is the biggest regressive delta, comprising 300,000 km2, with a sediment capacity of 500,000 km3 and a sedimentary layer of more than ten kilometers in the basin's depocenter (Kaplan et al. 1994; Kulke and Zantop 1995). The research area's boundary is identical to that of southern Nigeria's geology and south-western Cameroon, with a boundary to the north similar to that of the Benin border. Outliers of the Cretaceous on the Abakaliki high are marked by the Cretaceous on the Calabar border, an axis line bordered by the Precambrian (Tuttle et al. 1999).
Benin, Agbada, and Akata are the three major formations composed of rivers, coastal sandstones, interbedded sandstones and shales deposited in a transitional to marine environment, with huge marine shales. The Niger Delta basin has six to seven depobelts, which correlate to different periods of the delta's evolutionary history, beginning in the north (northern delta) and ending offshore. Individually, the depobelt is a self-contained entity in terms of sedimentation, structural deformation, hydrocarbon production and accumulation (Evamy et al. 1978; Faboya et al. 2018).
The petroleum system research studies the production, movement, accumulation, and entrapment of hydrocarbons (Magoon and Schmoker 2000). The most common method for analyzing the petroleum system in a particular area is to estimate the quantity of accumulated hydrocarbons in the prospect, the composition of its source rock, and the degree of thermal maturity of all organic elements (Aladwani 2021). The Niger Delta's petroleum system remains unclear despite extensive oil exploration and production operations in the region. Hydrocarbons typically exist in many productive strata at various depths within the basin, raising the question of whether the hydrocarbons are from the same source. Several source rock organofacies are suggested based on the distributions of light hydrocarbons and biomarker characteristics such as the oleanane/hopane ratio (Eneogwe and Ekundayo 2003). Based on the existence of pentacyclic triterpane skeletons of hopane and oleanane, as well as C27–C29 steranes in oil, the researchers revealed three unique families of Nigerian crude oil gathered from the Niger Delta depobelts. Sonibare et al. (2008) discovered that the oils were derived from both marine and terrestrial sources. Eneogwe and Ekundayo (2003) further determined the hydrocarbon route in the basin using maturity characteristics in the oils they analyzed. They concluded that as one proceeds from onshore to offshore, oil maturity decreases, indicating that either offshore oil was formed by younger source rocks or that first-generated oil from older sources moved largely to offshore areas.
Due to the possibility of investigating the diagenetic evolution pathways of chemical compounds, biomarkers can be used to describe organic matter’s origin and maturity, as well as the paleoenvironmental conditions (Peters et al. 2005). Biomarkers were extensively used to describe the organic matter’s origin and paleoenvironmental conditions within the lower and middle Jurassic strata from the epicontinental seas located in the Central European Basin System area (Ruebsam et al. 2020; Zakrzewski and Kosakowski 2021). Biomarkers are complex biological molecules formed from living organisms in oil that contain information about the oil. They are mostly derived from biological organisms that have been preserved over time (Hancok et al. 2013). These compounds are also known as genetic markers because they contain information on thermal history (maturity), the source of organic matter, and the paleoenvironmental deposition of the oils (Adedosu and Sonibare 2005).
This research was designed to examine and evaluate the distribution of biomarkers in selected crude oils from southern Nigerian producing fields in order to add to preceding research information on the organic matter origin, thermal maturity, and paleoenvironmental deposition of the crude oils based on information from geochemical evaluation from gas chromatography-mass spectrometry (GC–MS) analysis. The maturity status of oil from the southern region of the basin must be determined in order to gain a better understanding of the basin's petroleum system.
Geologic settings of study area
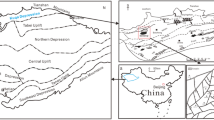
The geology of the research area, which is that of southern Nigeria, defines the Niger Delta basin's onshore regions and, in turn, the province's offshore boundaries to the eastern boundary of the Dahomey basin (Reijers 2011). It lies in the Gulf of Guinea (Fig. 1) and has three geologic formations: Benin, Agbada, and Akata (Haack et al. 2002).
The tertiary petroleum system, also known as the Akata-Agbada petroleum system, was established as the petroleum system of the Niger Delta (Fig. 2a, b). The Benin, Agbada, and Akata formations represent the three lithostratigraphic units, or formations, that make-up the basin of the region (Short and Stauble 1967; Doust and Omatsola 1990; Onojake et al. 2015). Benin (Oligocene), the youngest formation, is built of sandstone in a river and coastal environment, and under it is the Agbada (Eocene), which is made of interbedded sandstone and shale deposited in a transitional to marine environment. This formation's sandstone reservoir produces crude oil, and the Paleocene Akata formation is made up of a paralic sequence of interblended sands and large marine shales (Weber et al. 1978). The Agbada and Akata formations are prospective sources of crude oil in the Niger Delta region (Ekweozor and Daukoru 1994). The basin is categorized into 6–7 east–west bound units that correspond to depobelts.
a Geological map and sedimentary basins of Nigeria. b Description of the regional stratigraphy of the Niger Delta basin and the variable density-seismic display of the major stratigraphic sections (Corredor et al. 2005)
Stratigraphic column of the study area
The Niger Delta, which covers Southern Nigeria, is made up of three major strata that contribute petroleum habitat: the Akata, Agbada, and Benin formations. The first formation (Akata) is a marine-derived structure found at the delta's base. Furthermore, it extends from the Paleocene to the Recent, has tremendous pressure, and is beneath the delta. It comprises thick shale structures: prospective source rocks, marine sedimentary sand, deep water reservoirs, and a trace of clay and silt (Fig. 2a, b). Akata formation is associated with a low energy condition and a lack of oxygen, which were formed in low stands when terrestrial organic matter compositions and clays were conveyed to deep water regions (Stacher 1995).
The projected thickness of the formation, according to Doust and Omotsola (1990), is about 7000 m. The second formation (Agbada) is the main petroleum-bearing block, which is composed of sand and shale and dates from the Eocene to the Recent. The formation comprises paralic siliciclastic that is almost 3700 m thick and constitutes the deltaic section of the series. Shale and sandstone layers were generated in almost equal amounts in the lower Agbada formation. The upper region, on the other hand, is mostly sand with occasional shale interbeds. The Benin formation, which follows the Agbada formation, is a late Eocene to recent continental deposit of sedimentary and high coastal plain sands up to 2000 miters thick (Avbovbo 1978). The upper Benin formations consist of freshwater-bearing, continental rocks. The upper Benin formations consist of freshwater-bearing, continental sand, and gravel stone. From the late Eocene to the recent, the formation is a continental deposit of sedimentary and upper littoral plain sands with a thickness of up to 2000 m (Smith-Rouch 2006). Tectonic traps created during the stratigraphic deformation of the Agbada formation and geological traps formed preferentially along the delta border are the most notable reservoir formations in the study region (Niger Delta) (Beka and Oti 1995).
Materials and method
Sample collection and preparation
Four crude oil samples were obtained from three different oil fields in three different southern states of Nigeria, namely: Delta, Bayelsa, and Abia, respectively, in Teflon-caped glass vials and labeled as Kwale (KW), Kolo creek (KLC), Owaza (OWA1 and OWA2). Figure 1 shows the sample collection areas.
Fractionation of crude oil samples by column chromatography
Each of the oil samples was fractionated using column chromatography with petroleum ether (70 mL) as an eluent for aliphatic constituents and dichloromethane (DCM) (70 mL) as an eluent for aromatic constituents, respectively. The aliphatic fraction was recovered by carefully evaporating the solvent in a sand bath, followed by removal of the excess solvent with nitrogen gas (Farrimond et al. 1996).
Gas chromatography-mass spectroscopy analysis of the aliphatic fraction
The gas chromatography-mass spectrometry (GC–MS) was used to analyze the aliphatic hydrocarbon fraction to observe fragment ions of specific biomarkers with a standard Hewlett-Packard 5890II GC coupled to a split or split-less inlet/injector of 280 °C connected to a HP 5972 MSD (mass spectrometry detector) using a 70 eV electron voltage, a 220 A filament current, and a 160 °C source temperature. The collection of data was monitored by the HP Vectra PC chem station computer in full scan mode (FSM) and selected ion mode (SIM). 1µL of diluted sample was injected into the inlet using the auto-sampler HP 7376 (290 °C) and split open after 1 min. Component separation was achieved on a capillary column (30 m × 0.25 mm ID DB-5) sintered with silica and coated with 0.25 m (film thickness) of 5% phenylmethyl silicone (HP-5). The GC was set to operate at a temperature of 40/50–300 °C at a count of 4 °C per minute and then held for 16.7–20 min. Helium served as the carrier gas, flowing at 1.0 mL/min at 500 Kpa with a slit at 30 mL/min. The data was recorded on a digital audio tape (DAT), and the peak was integrated using the RTE integrator. Figure 3 is a flow chart showing the methodology used in the analysis of crude oils.
Results and discussion
The results of the peak identities calculated from the selected ion modes of the m/z 85, 191, and 217 saturated fractions of KW, KLC, OWA1, and OWA2 are shown in Table 1, 2 and 3, respectively. The chromatograms obtained from the GC–MS revealed that n-alkanes, acyclic isoprenoids, steranes, and terpanes (hopanes) were distributed in the crude oil samples. The peaks were identified, and the areas of the respective peaks were used to obtain values for standard plots of Pr/nC17 against Ph/nC18 and other cross-plots used for geochemical evaluation of the crude oil.
Parameters calculated from the aliphatic fraction of the samples for area deposition, source, and thermal maturity are shown in Tables 4 and 5, respectively. The chromatograms of m/z 191 and 217 showing the distribution of hopanes and steranes for the oils are shown in Fig. 4A–F.
A GC–MS chromatogram of m/z 85 of n-alkanes and isoprenoids distribution for crude oil sample KW. B GC–MS chromatogram of m/z 85 of n-alkanes and isoprenoids distribution of crude oil sample KLC. C GC–MS chromatogram of m/z 191 of hopanes for crude oil sample KW. D GC–MS chromatogram of m/z 191 of hopanes for crude oil sample OWA1. E GC–MS chromatogram of m/z 217 of the steranes for crude oil sample KW. F GC–MS chromatogram of m/z 217 of the steranes for crude oil sample OWA1
Thermal maturity
Thermal maturity emphasizes the effects of heat and time on sediment burial with the transformation of kerogen to petroleum due thermal exposure. Thermal maturity has been evaluated using a variety of variables and biomarkers, including vitrinite reflectance data (% Ro), carbon preference index (CPI), pentacyclic triterpenes, and regular sterane isomerization ratios (Moldowan et al. 1985). The Ts/(Ts + Tm) ratio (also known as Ts/Tm) can be employed to evaluate thermal maturity of oils (Seifert and Moldowan 1980; Abdullah et al. 2021). This ratio increases with the oil’s thermal maturity, but also may be affected by source lithology (Ekpo et al. 2012). In general, most oil samples have a ratio of diastereoisomers Ts less than Tm, and thus values of Ts/(Ts + Tm) are less than one. Table 5 shows the increasing trend of KW, KLC, OWA1 and OWA2 from 0.46 to 0.50 (Fig. 5), indicating incomplete isomerization of the compound, and the crude oils have reached the oil generating window inferring they are thermally mature (Peters and Moldowan 1993; Tissot and Welte 1984; Onojake et al. 2015; Fu et al. 2023).
The (22S/22)S + 22R) homohopane ratio is one of frequently utilized biomarker maturity indicator. During maturation, the C31 or C32 22S/22S + 22R homohopane ratios increase from zero to approximately 0.6 at equilibrium. Some researchers determined the maturity of lower Jurassic strata based on the CPI, C29 ββ/(αα + ββ), C29 20S/(S + R), C31 S/(S + R) (Ekpo et al. 2012; Zakrzewski et al. 2022). It is postulated that oil generation has just begun at values within the range of 0.50 to 0.54, but ratios between 0.57 and 0.62 indicate that the oil generation window has been reached. In this research, apart from KLC with a C32 homohopane isomerization value of 0.51 (Table 5), signifying it just barely entered the oil generation window, values for OWA1, OWA2 and KW, were 0.58 for both OWA1 and OWA2 and 0.59 for KW, which showed that these oils have reached the oil generation window and are thermally mature. The 20S/(20S + 20R) and ββ/(ββ + αα) C29 sterane ratios suggest a comparable explanation, with the moretane/hopane ratios. Thermal maturity causes the moretane fraction of its comparable hopanes to decrease from around 0.8 in immature sediments to roughly 0.05 to 0.15 in mature source rocks and oils (Connan et al. 1986).
The moretane/hopane ratio for the studied crude oils ranged from 0.20 to 0.8 to 0.21 for KW, 0.23 for KLC, 0.20 for OWA1 and 0.17 for OWA1. From the values obtained, it was observed that all the oils have some level of maturity, with OWA1 being the most mature. This confirms what various authors have said as regards the use of this ratio as an indicator for thermal maturity. From the data shown (Table 5), the Moretane/Hopane ratios were 0.21, 0.23, 0.17, and 0.20 for KW, KLC, OWA1, and OWA2. From the values obtained, it was observed that the oils all have some level of maturity, with the most mature being OWA1. Further confirmation that the crude oils have reached the beginning of the oil generating window.
Furthermore, thermal maturity was obtained from the ratios of cholestane (C29ααα 20S/(20S + 20R) and ββ/(ββ + αα) C29 sterane, which ranged from 0.28 to 0.66 and 0.33 to 0.71 signifying an oil generating window including a high level of maturity (Peters and Moldowan 1993; Seifert and Moldowan 1980; Tissot and Welte 1984; Huang et al. 2022).
Source of organic matter
The organic richness and composition of the organic matter input are the key parameters establishing the amount and type of petroleum formation in the source rocks (Aziz et al. 2020; Sohail et al. 2022). Once, thermal maturity is reached, the presence of adequate quantity of organic matter in the source rocks is crucial for managing the hydrocarbon generative potential (Aziz et al. 2020). The distribution pattern of steranes in oil can give information on the source of organic matter in the crude oil (Walples and Machihara 1991; Ahmad et al. 2022). Regular steranes/hopanes values are commonly employed to indicate eukaryote (mostly algae and advanced plants) and prokaryote (bacteria) inputs in crude oil parent materials. The high Σregular steranes/Σ hopanes (≥ 1) value represents the marine organic characteristics of algae; the low regular steranes/hopanes ratio, on the other hand, shows the characteristics of terrigenous organic matter or organic matter altered by bacteria (Ekpo et al. 2012; Younes 2001). The calculated sterane/hopane ratios (Table 5) ranged from 0.16 to 0.87, which is less than unity. The low ratio is a characteristic of terrigenous organic matter, or organic matter that must have undergone some modification by microorganisms (Connan et al. 1986).
Researchers such as Younes (2001) reported that Ph/nC18 > 0.30 is typical of shale source rocks, while oils of carbonate source rocks have Ph/nC18 < 0.30. Pr/nC17 ratio > 0.60 signifies terrestrial input to source rock. Also Pr/nC17 < 0.50 indicates marine matter input to source rock. Figure 6 shows that all oil samples have a Ph/nC18 greater than 0.30, typifying shale source rock, while the Pr/nC17 of oil samples OWA1 and OWA2 have Pr/nC17 ratios exceeding 0.60, signifying terrestrial input to source rock, while KW and KLC have Pr/nC17 ratios less than 0.50, which is characteristic of marine input to source rock (Uzoegbu et al. 2023).
Phytoplankton, bryophytes, and aquatic plants can all be associated with n-alkanes that are predominately mid-chain C21–C25 homologues (Volkman et al. 1998). Although, the high abundances of long-chain C27 homologues can be predominantly attributed to input from terrestrial plants (Zakrzewski et al. 2023). The ratio of isoprenoids to n-alkanes easily distinguishes oil families into marine organic matter contribution and suboxic-anoxic (Type II/III kerogens) depositional conditions for Family-A, which is mostly indicated by low pristane/phytane ratios and pristane/n-C17 ratios that are greater than or equal to phystane/n-C18 ratios. Pristane/phytane ratios that are slightly high and a correlation between pristane/n-C17 and phytane/n-C18 for Family-C point to the presence of mixed organic facies and suboxic depositional conditions. A Type II/III source rock with considerable terrestrial organic facies and dysoxicoxic depositional conditions can be determined by high pristane/phytane ratios and the pristane/n-C17 against phytane/n-C18 relationship for Family-B (Fig. 6) (Uzoegbu et al. 2023). The bituminite shale rocks contain Type II/III kerogen, which is both gas- and oil-prone and has a high potential for oil production (Hakimi et al. 2023).
Philip (2003) showed that ratios of C29/C30 hopanes are mostly high (> 1) in crude oils produced from organic-rich carbonates and evaporates and less than unity for oils from shale or carbonaceous source rocks. The oil samples under study have C29/C30 hopanes ranging from 0.60 to 0.67, which are all less than unity. Signifying that the oils might have come from carbonaceous organic matter-rich shale source rocks.
The presence of C29 moretane, gammacerance, oleananes, and a slight high concentration of C28 bisnorhopanes supports this proposition and designates an age range for the crude oils from cretaceous or younger and higher in tertiary sediments, which coincide with the evolution of angiosperms and are indicative of both source rock age and terrestrial input (Fazeelat et al. 2009; Peters et al. 2005; Onojake and Abrakasa 2021). The crude oil came from a shale source rock with terrigenous and marine inputs, according to the results of the various ratios used in this study.
Depositional environments
Since, some compounds are related to organisms or plants that thrive in specific forms of depositional environments, biomarkers can be used to indicate depositional paleoenvironments (Powel and Mckirdy 1973). Consequently, Powel and Mckirdy (1973) postulated a process for the synthesis of a very high ratio of pristane under oxic conditions and moderately high concentrations of phytane under reducing conditions. Crude oil and coal extract contain isoprenoids such as pristane (Pr) and phytane (Ph), and the ratio of pristane/phytane (Pr/Ph) is extensively utilized to evaluate depositional environments (Ten Haven et al. 1987). Values greater than one indicate oxic conditions, and values less than one (< 1) indicate anoxic paleodepositional environments (Ten Haven and Rullkötter 1988; Pandey et al. 2012). Consequently, the Pr/Ph ratio has evolved into an indicator of the toxicity of the depositional environment. Both pristane and phytane are generated from the phytol substituent of chlorophyll, either under reducing or oxidizing conditions. The pristane-to-phytane ratio is used to determine the redox state of sediments during diagenesis (Osuji and Anita 2005).
The pristane/phytane ratio is one of the most commonly used correlation parameters and has been utilized as an indication of the depositional environment (Eneogwe and Ekundayo 2003; Sletten 2003; Uzoegbu et al. 2023). A pristane/phytane ratio higher than 3.0 is thought to be associated with a terrigenous bed with oxidizing conditions (Lijmbach 1975). Ten Haven and Rullkötter (1988) stated that Pr/Ph ratios (> 3.0) suggest terrigenous input under oxic conditions, whereas low Pr/Ph (0.8) represents an anoxic, hypersaline, or carbonate environment. Terrestrial sediments often have high pr/ph ratios (greater than 3). Pr/ph ratios of 1 to 3 suggest an oxidizing depositional (Ekpo et al. 2018). According to Lijmbach (1975), low values (Pr/Ph < 2) indicate marine depositional environments such as marine, fresh, and brackish water (reducing conditions), values between two and four (2–4) indicate fluvio-marine and coastal swamp depositional environments, and high ratios up to ten (10) describe oxidizing conditions and peat/swamp depositional environments. The chromatogram of the samples showed a predominance of phytane over pristane, indicating an anoxic paleoenvironment, as phytane is formed by the reduction of the phytol side chain of the chlorophyll molecule (Powel and Mckirdy 1973). The Pr/Ph ratio of the four crude oil samples is shown in Table 4. KW and KLC had values of 0.34 and 0.42, respectively, suggesting anoxic, hypersaline, or carbonate environments, while the values for OWA1 and OWA2 were 0.89 and 0.80, respectively, which also point towards anoxic depositional environments. Though without corroborating data, Pr/Ph ratios of 0.8 to 3.00 are thought to designate certain paleoenvironmental conditions. The ratio of Pr/nC17 can be applied to distinguish organic matter generated in swamp environments (more than 0.1) from organic matter formed in marine environments (less than 0.5), although the ratio is influenced by maturity in addition to biodegradation (Osuji and Anita 2005). The calculated values for crude oil samples KW and KLC have values of 0.34 and 0.39 (< 0.5), while OWA1 and OWA2 have values of 0.87 and 1.0. The obtained results are typical of type II organic matter from shale in the marine environment, where values should be between 0.6 and 1.6 (Ekpo et al. 2012).
The cross-plot of Pr/nC17 vs. Ph/nC18, as shown in Fig. 6, is supportive evidence that the studied crude oils are classified into two types (A and B), produced from mixed organic matter with KW and KLC in the anoxic environment while OWA1 and OWA2 are in the oxic depositional environment (Huang and Meinschein 1979).
The computed relative abundance of C27, C28, and C29 steranes represents the percentage distributions of C27, C28, and C29 steranes for all samples (Table 5). C27 sterane of 41.2% and 33.6% for OWA1 and OWA2 predominated over C28 and C29, which is typical of organic origin with a marine input. KLC and KW, with dominant C28 and C29 of 58% and 38%, clearly show a mixed origin of marine and terrigenous plants owing to the high presence of oleanane in the chromatograms of both samples (Onojake and Abrakasa 2021).
The relative abundance of C27, C28, and C29 steranes, which ranged from 27.4 to 41.2, 26.3 to 58.7, and 32.0 to 34.0 percent, respectively (Table 5), indicates crude oil from source rock with mixed origins (terrestrial and marine) as shown in Fig. 7 (Su and Jiang 2022; Yu et al. 2022). Furthermore, the higher abundance of C27 steranes over C29 steranes suggests that more algal constituents were deposited into the source rock (Peters and Moldowan 1993; Hanson et al. 2000; Duan et al. 2008).
Ternary diagram of regular C27 – C29 steranes indicating the relationship between the organic matter input and depositional environment (modified after Huang and Meinschein 1979)
Conclusions
Biomarkers or genetic markers were used to investigate the geochemical characteristics (maturity, source of organic matter, depositional environment and genetic relationship) of crude oils from some fields in the Niger Delta basin, Nigeria using column chromatography and Gas chromatographic—mass spectroscopic (GC–MS) techniques. The conclusions from this study are summarized below:
-
1.
Organic geochemical evaluation of representative crude oils from southern Nigeria was carried out and the biomarker ratios and the different cross-plots served as tools for appraising the different oil samples geochemically.
-
2.
Each crude oil assay from different producing fields has its own distinct characteristics, ranging from barely reaching the oil generating window for some to actual thermal maturity for others.
-
3.
The distribution patterns of normal alkanes, hopanes, and steranes, as well as source-specific maturity and of biomarker diagnostic ratios, such as Pr/Ph, Pr/nC17, Ph/nC18, C29: (20S/(20S + 20R) and Ts/(Ts + Tm), revealed a high level of genetic similarity across the three producing fields. These information identified two genetically related crude oil families.
-
4.
This research also revealed that the origin of the crude oils was shale source rock containing mixed organic matter from terrigenous and marine input, showing hypersaline and anoxic environments of deposition.
-
5.
The biomarker maturity ratios revealed that the oils have reached the oil generation window and are thermally mature, with no clear evidence of biodegradation.
References
Abdullah ES, Ebiad MA, Rashad AM, El Nady MM, El-Sabbagh SM (2021) Thermal maturity assessment of some Egyptian crude oils as implication from naphthalene, phenanthrene and alkyl substituents. Egypt J Petrol 30(1):17–24. https://doi.org/10.1016/j.ejpe.2020.12.004
Abrakasa S, Ukaegbu V, Onojake M (2016) The source kitchen of the Niger Delta oils: case study on Nembe Creek E1.0 and the Kolo Creek E2.0 Reservoirs. J Petrol Expl Prod Technol 6(3):331–341. https://doi.org/10.1007/s13202-015-0211-9
Adedosu TA, Sonibare OO (2005) Characterization of Niger delta crude oil by infrared spectroscopy. J Appl Sci 5(5):906–909. https://doi.org/10.3923/jas.2005.906.909
Ahmed A, Khan T, Jahandad S, Hakimi MH, Gharib AF, Kahal AY, Khan MA, Munir MN (2022) Organic matter characteristics of the Datta Shale formation and their contribution to conventional oil generation resources in the Upper Indus Basin, Northern Pakistan. Asian J Earth Sci 224:104975. https://doi.org/10.1016/j.jseaes.2021.104975
Aladwani NS (2021) Assessment of petroleum system of Arabian Iranian Basin in Kuwait. All Earth 33(1):108–123. https://doi.org/10.1080/27669645.2021.1976930
Avbovbo AA (1978) Tertiary lithostratigraphy of Niger delta. AAPG Bull 62(2):295–300. https://doi.org/10.1306/c1ea482e-16c9-11d7-8645000102c1865d
Aziz H, Ehsan M, Ali A, Khan HK, Khan A (2020) Hydrocarbon source rock evaluation and quantification of organic richness from correlation of well logs and geochemical data: a case study from the Sembar formation, Southern Indus Basin, Pakistan. J Nat Gas Sci Eng 81:103433. https://doi.org/10.1016/j.jngse.2020.103433
Beka FT, Oti MN (1995) The distal offshore Niger Delta: frontier prospects of a mature petroleum province. In: Geol of deltas, pp 237–241. https://doi.org/10.4236/ajcm.2014.42010
Connan J, Bouroullec J, Dessort D, Albrecht P (1986) The microbial input in carbonate-anhydrite facies of a sabkha palaeoenvironment from Guatemala: a molecular approach. Org Geochem 10(1–3):29–50. https://doi.org/10.1016/0146-6380(86)90007-0
Corredor F, Shaw JH, Bilotti F (2005) Structural styles in the deep-water fold and thrust belts of the Niger Delta. AAPG Bull 89(6):753–780. https://doi.org/10.1306/02170504074
Doust H, Omatsola E (1990) Niger delta divergent/passive margin basins. AAPG Bull 45:201–238. https://doi.org/10.1306/m48508c4
Duan Y, Wang CY, Zheng CY, Wu BX, Zheng GD (2008) Geochemical study of crude oils from the Xifeng oilfield of the Ordos basin, China. J Asian Earth Sci 31(4–6):341–356. https://doi.org/10.1016/j.jseaes.2007.05.003
Ekpo BO, Ibok UJ, Essien N, Wehner H (2012) Geochemistry and organic petrography of Cretaceous sediments of the Calabar Flank, Southeastern Nigeria. Mar Petrol Geol 35(1):252–268. https://doi.org/10.1016/j.marpetgeo.2012.03.010
Ekpo BO, Essien N, Neji PA, Etsenake RO (2018) Geochemical fingerprinting of western offshore Niger Delta oils. J Petrol Sci Eng 160:452–464. https://doi.org/10.1016/j.petrol.2017.10.041
Ekweozor CM, Daukoru EM (1994) Northern Delta Depobelt Portion of the Akata-Agbada (!) Petroleum System, Niger Delta, Nigeria: Chapter 36: Part VI. Case Studie—Eastern AAPG Memoir Hemisphere 60:599–614. https://doi.org/10.1306/m60585c36
Eneogwe C, Ekundayo O (2003) Geochemical correlation of crude oils in the NW Niger Delta, Nigeria. J Petrol Geol 26(1):95–10. https://doi.org/10.1111/j.1747-5457.2003.tb00019.x
Energy Information Administration (EIA) (2015).Country analysis brief: Nigeria. http://www.eia.gov/beta/international/analysis_includes/countries_long/Nigeria/nigeria
Evamy BD, Haremboure J, Kamerling P, Knaap WA, Molloy FA, Rowlands PH (1978) Hydrocarbon habitat of Tertiary Niger delta. AAPG Bull 62(1):1–39. https://doi.org/10.1306/c1ea47ed-16c9-11d7-8645000102c1865d
Faboya OL, Ojo AA, Bello H, Osasona I (2018) Geochemical investigation of trace elements in crude oils from two different depobelts in the Niger Delta basin, Nigeria. Petrol Sci Technol 36(2):130–135. https://doi.org/10.1080/10916466.2017.1410557
Farrimond P, Bevan JC, Bishop AN (1996) Hopanoid hydrocarbon maturation by an igneous intrusion. Org Geoch 25(3–4):149–164. https://doi.org/10.1016/s0146-6380(96)00128-3
Fazeelat T, Asif M, Saleem A, Zulfiqar MA, Nadeem S (2009) Geochemical investigation of crude oils from different oils fields of the Potwar Basin. J Chem Soc Pak 31(6):863–870
Fu J, Li M, Sun Y, Li Y, Yang Y, Lu X, Lai GT (2023) Thermal maturity of crude oils in the Baiyun sag, Pearl River Mouth basin, South China Sea: insights from biomarkers, aromatic hydrocarbon and adamantane compounds. Mar Petrol Geol. https://doi.org/10.1016/j.marpetgeo.2023.106353
Haack RC, Sundararaman P, Diedjomahor JO, Gant NJ (2002) Niger delta petroleum systems: regional geology, organic facies and thermal maturity. In: AAPG annual meeting. https://doi.org/10.1306/61eeea0c-173e-11d7-8645000102c1865d
Hakimi MH, Kumar A, Singh AK, Lashin A, Rahim A, Varfolomeev MA, Mustapha KA (2023) Geochemistry and organic petrology of the bituminite shales from the Kapurdi mine, Rajasthan of NW India: implications for waxy oil generation potential. J Petrol Expl Prod Technol 13(2):505–521. https://doi.org/10.1007/s13202-022-01597-9
Hanson AD, Zhang SC, Moldowan JM, Liang DG, Zhang BM (2000) Molecular organic geochemistry of the Tarim Basin, northwest China. AAPG Bull 84(6):1109–1128. https://doi.org/10.1306/a9673c52-1738-11d7-8645000102c1865d
Huang WY, Meinschein WG (1979) Sterols as ecological indicators. Geochim Cosmochim Acta 43(5):739–745. https://doi.org/10.1016/0016-7037(79)90257-6
Huang H, di Primio R, Pedersen JH, Silva R, Algeer R, Ma J, Larter S (2022) On the determination of oil charge history and the practical application of molecular maturity markers. Mar Petrol Geol 139:105586. https://doi.org/10.1016/j.marpetgeo.2022.105586
Kaplan A, Lusser CU, Norton IO (1994) Tectonic map of the world, panel 10”. American Association of Petroleum Geologists, AAPG Tulsa. OCLC Number/Unique Identifier: 223027395
Kulke H, Zantop H (1995) Regional petroleum geology of the world. Part I: Europe and Asia. Part II: Africa, America, Australia and Antarctica. Econ Geol Bull Soc Econ Geol 90(5):1354. https://doi.org/10.4236/jhepgc.2021.71014
Lijmbach WM (1975) SP (1) On the Origin of Petroleum. In: 9th world petroleum congress. Onepetro. WPC-16134
Magoon LB, Schmoker JW (2000) The total petroleum system–the natural fluid network that constrains the assessment unit. US Geol Surv World Petrol Assess. https://doi.org/10.3133/ds60
Moldowan JM, Seifert WK, Gallegos EJ (1985) Relationship between petroleum composition and depositional environment of petroleum source rocks. AAPG Bull 69(8):1255–1268. https://doi.org/10.1306/ad462bc8-16f7-11d7-8645000102c1865d
Onojake MC, Abrakasa S (2021) The occurrence and distribution of Oleanane biomarkers in crude oils as an index. J Petrol Sci Technol 11(1):43–50. https://doi.org/10.22078/jpst.2021.4248.1690
Onojake MC, Osuji LC, Abrakasa S (2015) Source, depositional environment and maturity levels of some crude oils in Southwest Niger Delta, Nigeria. Chin J Geochem 34:224–232
Osuji LC, Antia BS (2005) Geochemical implication of some chemical fossils as indicators of petroleum source rocks. J Sci Environ Mgt 9(1):45–49
Pandey IP, Joshi HC, Tyagi A, Tiwari S, Garg N (2012) Study of the parameters and biomarkers of crude oils. Adv Pure Appl Chem 1(3):49–52
Peters KE, Moldowan JM (1993) The biomarker guide: interpreting molecular fossils in petroleum and ancient sediments. Prentice-Hall, Englewood Cliffs, pp 103–114
Peters KE, Peters P, Walters CC, Moldowan JM (2005) The biomarker guide, vol 1. Cambridge University Press, Cambridge. https://doi.org/10.1017/S0016756806212056
Philp RP (2003) Formation and geochemistry of oil and gas. In: Holland HD, Turekian KK (eds) Treatise on geochemistry sediments, diagenesis and sedimentary rocks, vol 17. Elsevier, Amsterdam. https://doi.org/10.1016/B0-08-043751-6/07135-8
Powell TG, McKirdy DM (1973) Relationship between ratio of pristane to phytane, crude oil composition and geological environment in Australia. Nat Phys Sci 243(124):37–39. https://doi.org/10.1038/physci243037a0
Reijers TJA (2011) Stratigraphy and sedimentology of the Niger Delta. Geologos 17(3):133–162. https://doi.org/10.2478/v10118-011-0008-3
Ruebsam W, Pieńkowski G, Schwark L (2020) Toarcian climate and carbon cycle perturbations its impact on sea-level changes, enhanced mobilization and oxidation of fossil organic matter. Earth Planet Sci Lett 546:116417. https://doi.org/10.1016/j.epsl.2020.116417
Seifert WK, Moldowan JM (1980) The effect of thermal stress on source-rock quality as measured by hopane stereochemistry. Phys Chem Earth 12:229–237. https://doi.org/10.1016/0079-1946(79)90107-1
Short KC, Stauble AJ (1967) Outline of geology of Niger delta. AAPG Bull 51:761–779
Sletten EB (2003) A Comparison of petroleum from reservoirs and petroleum inclusions in Authigenic mineral Cements-Haltenbanken (Master's Thesis). University of Oslo, Department of Geology, pp 80–107. http://hdl.handle.net/10852/12344
Smith-Rouch LS (2006) Oligocene–Miocene Maykop/Diatom Total Petroleum System of the South Caspian Basin Province, Azerbaijan, Iran, and Turkmenistan. US Geol Surv Bull 2201:1–27
Sohail GM, Radwan AE, Mahmoud M (2022) A review of Pakistani shales for shale gas exploration and comparison to North American shale plays. Energy Rep 8:6423–6442. https://doi.org/10.1016/j.egyr.2022.04.074
Sonibare O, Alimi H, Jarvie D, Ehinola OA (2008) Origin and occurrence of crude oil in the Niger delta, Nigeria. J Petrol Sci Eng 61(2–4):99–107. https://doi.org/10.1016/j.petrol.2008.05.002
Stacher P (1995) Present understanding of the Niger Delta hydrocarbon habitat. In: Geology of deltas, pp 257–267. https://www.researchgate.net/publication/281508240
Su S, Jiang Y (2022) Biomarker geochemistry of paleogene crude oils and source rocks from the Raoyang Sag, Bohai Bay Basin, NE China: an oil-source rock correlation study. J Petrol Geol 45(1):57–77
Ten Haven HL, Rullkötter J (1988) The diagenetic fate of taraxer-14-ene and oleanene isomers. Geochim Cosmochim Acta 52(10):2543–2548. https://doi.org/10.1016/0016-7037(88)90312-2
Ten Haven HL, De Leeuw JW, Rullkötter J, Damsté JS (1987) Restricted utility of the pristane/phytane ratio as a palaeoenvironmental indicator. Nature 330(6149):641–643. https://doi.org/10.1038/330641a0
Tissot BP, Welte DH (1984) Petroleum formation and occurrence, 2nd edn. Springer, Berlin
Tuttle ML, Charpentier RR, Brownfield ME (1999) The Niger delta petroleum system: Niger delta province, Nigeria, Cameroon, and equatorial guinea, Africa, US Department of the Interior, US Geol Surv 50-99
Uzoegbu U, Madu AJ, Ugwueze U, Omang O (2023) API gravities and geochemical evaluation of crude oils from sapele, niger–delta, nigeria. Global J Geol Sci 21(1):69–89. https://doi.org/10.4314/gjgs.v21i1.6
Volkman JK, Barrett SM, Blackburn SI, Mansour MP, Sikes EL, Gelin F (1998) Microalgal biomarkers: a review of recent research developments. Org Geochem 29(5–7):1163–1179
Walples DW, Machihara T (1991) Biomarkers for geologists, american association of petroleum geologist’s methods. In: Exploration series, vol 9, 91pp. https://doi.org/10.1017/S0016756800008529
Weber KJ, Mandl GJ, Pilaar WF, Lehner BVF, Precious RG (1978) The role of faults in hydrocarbon migration and trapping in Nigerian growth fault structures. In Offshore Technol Conf. OnePetro, Houston. OTC-3356-MS. https://doi.org/10.4043/3356-MS
Younes MA (2001) Application of biomarkers and stable carbon isotopes to assess the depositional environment of source rocks and the maturation of crude oils, East Zeit Field, southern Gulf of Suez. Egypt Petrol Sci Technol 19(9–10):1039–1061. https://doi.org/10.1081/LFT-100108293
Yu Y, Peng W, Shi S, Sun H, Lu M, Shang P, Lu Y (2022) Geochemical characterizations of source and depositional environment of hydrocarbons in the Lake Albert Rift Basin. Acta Geol Sin Eng Ed 96(1):261–272. https://doi.org/10.1111/1755-6724.14862
Zakrzewski A, Kosakowski P (2021) Impact of palaeo-wildfires on higher plant parameter revealed by new biomarker indicator. Palaeogeogr Palaeoclimatol Palaeoecol 579:110606. https://doi.org/10.1016/j.palaeo.2021.110606
Zakrzewski A, Waliczek M, Kosakowski P, Pańczak J (2022) Lower Jurassic in the central part of the Polish Basin-Geochemical and petrological approach. Mar Pet Geol 146:105922. https://doi.org/10.1016/j.marpetgeo.2022.105922
Zakrzewski A, Krajewski M, Kosakowski P (2023) Geochemical fingerprint—inter-basin chemostratigraphic correlation allowed for a more detailed stratigraphic subdivision of the Pliensbachian-Bathonian strata from the Carpathian Foredeep basement (Ukraine). Int J Earth Sci 112(5):1389–1412. https://doi.org/10.1007/s00531-023-02311-8
Acknowledgements
The researchers are immeasurably grateful to the Nigerian Department of Petroleum Resources (DPR), including various operators of the various producing fields, for authorization and access to the oil samples used in this investigation. The employees of Giolee Global Resources Limited in Port Harcourt, Rivers State, deserve special thanks for their invaluable contribution in providing the equipment for the laboratory study of crude oil samples. The effort of Mr. E. Akpan is also acknowledged for helping us with the required logistics that led to the collection of the oil samples. The authors thank Dr. George U. Ozulu for providing expert advice on the technical aspects of the article especially on sedimentology and stratigraphy of the study area.
Funding
The authors did not receive support from any organization for the submitted work during the preparation of this manuscript and conducting research for this study.
Author information
Authors and Affiliations
Contributions
MCO: Conceived, designed, supervised and was involved in the writing of the manuscript.NEN: Collected samples, analyzed interpreted result and wrote part of the manuscript. JOO: Collected samples, contributed reagents, analyzed and interpreted results. DAA: Contributed reagents, provided analysis tools and offered special advice. IO: Performed the experiments and interpreted part of the results. LCO: Provided expert advice and corrected the technical aspects of the article.
Corresponding author
Ethics declarations
Conflict of interest
Authors hereby declare that no competitive interests exist.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Onojake, M.C., Nkanta, N.E., Osakwe, J.O. et al. Organic geochemical evaluation of crude oils from some producing fields in the Niger Delta basin, Nigeria. J Petrol Explor Prod Technol (2024). https://doi.org/10.1007/s13202-024-01799-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13202-024-01799-3