Abstract
The purpose of this study was to observe how the composition of organic matter (OM) and the extent of anoxia during deposition within the Marcellus Formation in New York varied by distance from the sediment source in eastern New York. Lipid biomarkers (n-alkanes and fatty acids) in the extractable organic component (bitumen) of the shale samples were analyzed, and proxies such as the average chain length (ACL), aquatic to terrestrial ratio (ATR) and carbon preference index (CPI) of n-alkanes were calculated. Fatty acids were relatively non-abundant due to the age of the shale bed, but n-alkane distributions revealed that the primary component of the OM was terrigenous plants. The presence of shorter n-alkane chain lengths in the samples indicated that there was also a minor component of phytoplankton and algal (marine) sourced OM. Whole rock analyses were also conducted, and cerium anomalies were calculated as a proxy for anoxia. All samples had a negative anomaly value, indicating anoxic conditions during deposition. Two samples, however, contained values close to zero and thus were determined to have suboxic conditions. Anoxia and total organic matter (TOM) did not show any spatial trends across the basin, which may be caused by varying depths within the basin during deposition. A correlation between nickel concentrations and TOM was observed and indicates that algae was the primary source of the marine OM, which supports the lipid biomarker analysis. It was determined that the kerogen type of the Marcellus Formation in New York State is type III, consistent with a methane-forming shale bed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The Marcellus Formation, a Middle Devonian black shale, is well known as a prolific source for unconventional gas extraction, being targeted on the east coast of the USA (Kargbo et al. 2010). Gas potential is directly related to the type and amount of organic material within the sediment (Didyk et al. 1978). Black shale beds have a range of depositional facies and are known to vary widely in geochemical characteristics at different locations and depths, including in the amount and type of organic material present (Miceli and Philp 2012). Understanding how organic matter (OM) has been deposited, preserved and thermally altered in the Marcellus Formation and how these processes vary across New York could help target potential areas of high gas productivity. The purpose of this study is to characterize the OM within the Marcellus by analyzing distributions of chemical biomarkers in the solvent-extractable pool (bitumen) and examine how the OM varies with increasing distance from the sediment source (east–west), the degree of anoxia during deposition, and thermal maturation within the formation. The carbon preference index (CPI), average chain length (ACL), and aquatic to terrestrial ratio (ATR) of n-alkane biomarkers will be used to characterize the nature of the OM in the formation. Cerium anomalies will be used to determine the degree of anoxia within the basin.
Geologic setting
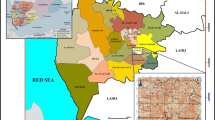
The Marcellus Formation was deposited in the Middle Devonian during the Acadian orogeny in the Appalachian foreland basin that formed on the Eastern USA (Fig. 1). During this time, the Appalachian basin was also subsiding from tectonic loading of the Acadian Mountains. This subsidence, combined with sea level rise, resulted in a deep basin consisting of anoxic conditions (Griffing and Ver Straeten 1991). While this is widely accepted as the depositional conditions for the Marcellus Formation in New York, Smith, Schieber, and Wilson, (2019) suggest shallow, oxygenated water for the Appalachian basin in New York consisting of benthic life. While their model goes against previous interpretations, it is the most recent sedimentological research conducted on the Marcellus Formation in New York and deserves to be mentioned as a possible alternative for Appalachian basin depositional conditions in New York during this time. Sediments eroding from the Acadian Mountains were transported via the Catskill Delta and deposited into the Appalachian basin (Mazzullo, 1973, Driese et al. 1997). The Marcellus Formation is therefore thicker in Eastern New York and thinner to the west (Lash and Engelder 2011). In New York, the Marcellus formation outcrops roughly parallel to the coast of Lake Ontario, dipping roughly two degrees toward the south into Pennsylvania (Fig. 1). The Marcellus Formation overlies the Onondaga Limestone, which is composed of patch reefs, and the boundary between the two formations marks the time when the basin subsidence and sea level rise begins (Griffing and Ver Straeten 1991). The Skaneateles Formation overlies the Marcellus Formation and signifies subsequent sea-level lowering and the return to oxic conditions (Werne et al. 2002).
Structure map of the Marcellus Formation. In New York, Pennsylvania, Maryland, Virginia, West Virginia, and Ohio. The map was acquired from the U.S. Energy Information Administration, and contains data from DrillingInfo Inc., New York State Geological Survey, Ohio State Geological Survey, Pennsylvania Bureau of Topographic & Geologic Survey, West Virginia Geological & Economic Survey, and U.S. Geological Survey. Production wells from January 2003 through December 2014 are used in the map. The contour lines represent the elevation of the Marcellus Formation top with a 1000-foot contour interval (Popova, et al., 2015, April 1)
Three members make up the Marcellus Formation: the Union Springs Shale, which is the deepest, the Cherry Valley Limestone, and the Oatka Creek Shale which is the uppermost member (Fig. 2). The Union Springs and the Cherry Valley Limestone members, however, are not observed in a portion of western New York (both being absent in almost the same locations), while the Oatka Creek shale is present across the entire state (Lash and Engelder 2011). In Central and Western New York, portions of the Cherry Valley Limestone and the Upper part of the Union Springs member are difficult to distinguish, which has led to incorrect thickness values for the Cherry Valley Limestone (Griffing and Ver Straeten 1991). Both the Union Springs and the Oatka Creek members have been targeted for hydrocarbons.
A general stratigraphic column of the stratigraphy of New York State from the Upper Ordovician to the Upper Devonian, modified from (Nyahay et al., 2007). Note that while the Marcellus is the most well-known black shale, New York also contains the Utica and Geneseo Shales
Map of sample locations which closely follows the outcrop pattern of the Marcellus Formation, M2 and M3 are quarry cuts taken from Callanan Quarry and Seneca Stone Quarry, respectively, while the rest of the samples are taken from outcrops and road cuts. Image from Google Earth Pro V 7.3.3.7786. (December 2016). New York, USA. 42°42′34.37’’ N, 76°51′08.66’’W Eye alt 202.51 mi. NOAA. Google 2020 [Accessed July 28th, 2020
Background
Multiple studies have been conducted on the gas potential of black shales by using biomarker ratios to determine characteristics of the OM (Ayyıldız et al. 2019, Agrawal and Sharma 2018, Gao et al. 2020, Miceli and Philp 2012, Qu et al. 2017). Biomarkers, classes of organic compounds with a known source, have been used to determine the gas potential of black shales because of the amount of information they contain related to OM source and depositional environment (Bianchi et al. 2016). Preserved compounds can be indicative of a marine or terrigenous OM origin, inputs from specific types of algae, plants and bacteria, and the degree of maturation that the OM within a source rock has undergone (Meyers 1997, Mani et al. 2017). The usefulness of biomarkers and other indicators to determine the potential of source rocks around the world has been demonstrated by Gao et al. (2020), Qu et al. (2017) and many others. While biomarkers may degrade due to diagenesis (Tissot et al. 1987, Mißbach et al. 2016), biomarkers such as n-alkanes are more resistant to changes caused by these processes (Meyers 1997), and have long been used to determine the source and maturity of kerogen (Bray and Evans 1961, Peters et al. 2005).
Multiple studies on the Marcellus Formation have been conducted across eastern North America to determine gas potential. Obermajer et al. (1997) evaluated the Marcellus in Ontario, Canada, and determined the source of the OM to be marine and the kerogen as type II. The deposition of large amounts of OM to this region of the Marcellus was from high productivity of marine plankton, as well as anoxic conditions in bottom water which increased preservation. This organic material was deposited in a shelf environment that was transitioning to a basin setting, and the reduction of dissolved oxygen was caused by a lack of circulation within the basin. Optical properties evaluated in Obermajer et al. (1997) showed that the Marcellus reached the early stages of oil formation, which was also supported by biomarker ratios. This study further validates the use of biomarkers in mature shales, and it will allow for a comparison in the variability of the Marcellus Formation.
There are various types of biomarkers that have been utilized to evaluate organic material to gain insight in the source of organic material, thermal maturation of source rocks, and other basin characteristics. One type of biomarker is n-alkanes. These compounds are hydrogen saturated, straight chain hydrocarbon, usually referred to by chain length (Kostecki et al. 2005), and commonly preserved within the organic matter found in shales. These organic compounds come from bacteria, archaea, and eukaryotes. They are also produced by the breakdown of biolipids such as fatty acids, esters, alcohols, alkenes, plant waxes and polymethylenic biopolymers (Grice and Eiserbeck 2013). Since these organic compounds and biolipids have a specific organic signal, the concentration of certain n-alkanes found in the organic material of a shale can be used to determine what was the source of organic material (e.g., marine or terrestrial). This information can then be further used to determine basin conditions.
Other basin characteristics can be determined by looking at trace elements within the sediments. Ratios of these trace metals may give vital pieces of information to help understand the depositional environment, such as anoxia, and other basin characteristics (Quinby-Hunt et al. 1989). Lewan and Maynard (1982) discuss the sources of enrichment of nickel and vanadium within organic-rich rocks and what these enrichments indicate about the depositional environment, providing important information when determining how a basin varies with respect to OM. Nickel enrichment in black shales was determined to be caused by algae absorbing nickel within the ocean water (Lewan and Maynard 1982). Chen and Sharma (2016) also determined that there is variability in concentrations of trace metals such as U, Mo, and V in organic-rich sections of the Marcellus Formation due to oscillations in redox conditions during deposition.
Werne et al. (2002) used a combined trace metal and geochemical proxy approach in the Oatka Creek member, the upper member of the Marcellus Formation in Western New York, to determine characteristics of the depositional environment. They also successfully used proxies such as the ratio of molybdenum to titanium (Mo/Ti), iron to titanium (Fe/Ti), and the concentration of organic carbon (Corg) to determine sea level during deposition. The investigation determined that redox indicators are more prominent in the Oatka Creek member because of sea level rise restricting the deposition of carbonate muds to the basin. It was also determined that euxinic conditions existed during deposition due to sea level rise.
Objectives
The purpose of this study is to characterize the OM within the Marcellus by analyzing distributions of chemical biomarkers in the solvent-extractable pool (bitumen) and examine how the OM varies with increasing distance from the sediment source (east–west), the degree of anoxia during deposition, and thermal maturation within the formation. The carbon preference index (CPI), average chain length (ACL), and aquatic to terrestrial ratio (ATR) of n-alkane biomarkers will be used to characterize the nature of the OM in the formation. Cerium anomalies will be used to determine the degree of anoxia within the basin.
Methodology
Sampling
Seven samples of the Marcellus Formation were obtained from outcrops along an east–west transect from Richfield Springs, NY to Leroy, NY (Fig. 2). Five samples were taken from road cuts. Two samples (M2, and M3) were collected from Callanan Quarry and the Seneca Stone Quarry, respectively. All seven samples are used in the inorganic analysis; however, due instrumental error, the data for sample four (M4) are not reported in the organic analysis.
Shale samples were trimmed by hand to obtain an unweathered sample. The unweathered inner core of the original sample was broken into smaller chips, which were pulverized using a Spex Mixer Mill 8000 for 45 min to achieve particles less than 270-mesh (Fig. 3).
Lipid analysis (biomarkers)
Lipid analysis procedures were modified from Canuel and Martens (1993). Roughly 1.5 g of sample was placed in steel vials and surrounded by combusted sand. OM was extracted using a Dionex Accelerated Solvent Extraction 150 system (ASE) with 65% dichloromethane (DCM) and 35% methanol (MeOH) by volume at 80 °C and ~ 1600 psi.
The lipid isolation procedure was modified from Bligh and Dyer (1959). The volumes of solvent in the ASE vials were adjusted to a final 1:1:0.9 solution of DCM/MeOH/20%NaCl in water. The solvent mixtures were then poured into 250-mL separatory funnels, shaken for 1 min, and allowed to stand for 1 h. Two layers formed: an organic layer containing the lipids in the bottom layer, and an aqueous layer on top. The organic layer was then drained and kept. 60 mL of hexane was then added to each separatory funnel, which were again shaken for 1 min and allowed to stand for 1 h. After separation, the top organic layer containing the lipids was added to the first organic fraction. The combined fractions were concentrated using rotary evaporation to ~ 10 mL, transferred to pre-weighed vials, and evaporated under a steady stream of N2 until dry. The residue is considered the total lipid extract (TLE). The final weight of the vials was measured, and the weight difference was used to estimate percent total lipids by weight in each sample.
The TLEs were dissolved using 100 µL of hexane, and the solution was transferred to 15-ml centrifuge tubes and evaporated to dryness with N2. Next, 3 mL of potassium hydroxide was added to each tube, which were then sealed under N2 and placed in the oven for saponification at 110 °C for 2 h.
To isolate n-alkane hydrocarbons, 5 mL of hexane and 3 mL of pure (Milli-Q) water were added to the saponified mixtures, which were shaken for 1 min and allowed to stand for 1 h. The top organic layer was pipetted out and transferred into another set of 15-mL centrifuge tubes. Another 5 mL of hexane was added to the original vials, which were again shaken for 1 min and allowed to stand for 1 h, and the top organic layer was also added to the new set of vials containing the first extract.
To isolate fatty acids, 3 mL of 3 M HCl were added to the saponified aqueous layer left over after n-alkane extraction and were shaken for 1 min and let stand for 1 h. The top organic layer was transferred into an empty vial. This was then repeated with another 5 ml of hexane, combining both extracts.
GC/MS analysis
The fatty acid and n-alkane extracts were dried under N2, reconstituted into ~ 150ul hexane, and injected onto an Agilent 7890a/5979 gas chromatograph/mass spectrometer equipped with either a DB/WAX-ETR column (for fatty acids) or a DB-5 column (for n-alkanes). Fatty acid data are not reported due to few compounds above the limit of detection. N-alkanes were identified by comparison to an external standard containing a range of even-chained n-alkanes, as well as by comparison with published mass spectra. m/z 57 peaks were integrated to determine compound abundances, as these ions showed minimal overlap with co-eluting compounds and were the most abundant ions in the n-alkane spectra. The ratio of a compound’s m/z 57 peak area to its concentration in the standard solution was used to correct its integrated peak area in a sample, thus correcting for differences in m/z 57 ion production. Due to a lack of known extraction efficiencies, absolute concentrations are not reported. Instead, percent relative abundances of each compound are calculated from the ratio of all normalized m/z 57 peak areas in a sample.
Organic matter
The OM content of each sample was determined by loss on ignition (LOI) (Xie, Dearing, and Blomendal 2000). Approximately five grams of powdered sample was placed in a massed crucible and dried at 100 °C to constant weight. After drying, the samples were ignited in an oven at 550 °C for 4 h. Loss on ignition was determined by comparing weights before and after ignition, and reported as percent total organic matter (%TOM).
Whole rock analysis
Whole rock analyses were completed by ACTLABS located in Ancaster, Ontario following method Lithogeochemistry Total IDENT 4E-expl. The following trace metals were determined by Instrumental Neutron Activation Analysis (INAA): As, Au, Br, Ce, Co, Cr, Cs, Eu, Hf, Hg, Ir, La, Lu, Mo, Nd, Rb, Sb, Sc, Se, Sm, and Ta. Multi-element INAA and total digestion inductivity coupled plasma (MULT INAA / TD-ICP) were used for Ag, and MULT INAA/ fusion inductivity coupled plasma (FUS-ICP) was used for Ba. TD-ICP was used for Ba, Bi, Cd, Cu, Ni, S, Zn, while Be, Sr, V, Y, Zr, Al2O3, CaO, Fe2O3, K2O, MgO, MnO, Na2O, P2O5, SiO2, TiO2, and LOI were analyzed by FUS-ICP.
Biomarker/Trace metal proxy formulas
ACL was calculated according to Eq. 1 (Poynter and Eglinton 1990):
where Cn is the relative abundance of the specified n-alkane and n is the number of carbons within each n-alkane. Only chain lengths 15 to 33 are used in the equation. The ACL indicates the average chain length (the average amount of carbon atoms) of the n-alkanes within each sample. Organic compounds that are marine sourced have lower ACL values from chain lengths 14 to 24 (Meyers and Ishiwatari, 1993), while terrestrial sourced organic compounds have higher ACLs that range from 21 to 39 (Eglinton and Hamilton 1967, Jeng 2006).
The ATR characterizes the relative amounts of marine and terrestrially sourced OM. The equation is the inverse of Meyers (1997) terrigenous to aquatic ratio (TAR) and is calculated as:
where Cn is the relative abundance of the specified n-alkane and n is the number of carbons within each n-alkane. Values less than 1 indicate terrestrial dominated OM, and values greater than one indicate marine dominated OM.
The CPI compares the ratio of odd to even n-alkanes and is used to help determine both the source of OM and the extent of thermal maturation (Bray and Evans 1961). High CPI ~ 5–10 indicates terrestrial sourced organic material; however, this value will become closer to 1.0 if the organic material has undergone more maturation (El Nemr et al. 2016, Theresa et al. 2018). Marine microorganisms and recycled organic matter will also result in CPI values close to one (Kennicutt II et al. 1987). Petrogenic input will have a CPI less than one (El Nemr et al. 2016). The CPI is calculated as follows, from Bray and Evans (1961):
where Cn is the relative abundance of the specified n-alkane of chain lengths 16 to 32.
Cerium (Ce) anomalies indicate the amount of anoxia within a basin during deposition. In rare earth element (REE) calculations, REE concentrations are normalized to a standard. It is then assumed that REE concentrations decline with increasing atomic number. The Ce anomaly is determined by the divergence of Ce concentrations from this trend which is determined by comparing concentrations to the adjacent REE (La and Pr in this circumstance). Ce is used for the anomaly because other than europium, it is the only rare earth element that is redox sensitive in depositional environments (Quinby-Hunt et al. 1989). So, the preservation of cerium can be used as a proxy for anoxic conditions according to the following equation from Quinby-Hunt et al. (1989):
where Ce is the cerium concentration, La is the lanthanum concentration, and Pr is the praseodymium concentration in each sample, all of which were normalized to chondrites. Negative values indicate anoxic conditions, while positive values indicate oxic conditions. Values that are less negative indicate less anoxic conditions (Quinby-Hunt et al. 1989).
Results
Total organic matter (%TOM) in the shales ranged from 2.43% to 14.46% (Table 1). Along the sampling transect, some spatial variability was seen (Fig. 6, Table 5) with the highest %TOM concentrations in sample M2: approximately twice as high as the next highest concentration. The lowest concentrations are found in samples M1, M5 and M7 (Table 2).
The percent total lipids by weight in the shale samples ranged from 0.98% to 1.96% and appear to generally increase east across the state (Fig. 4, Table 4). n-Alkanes were found with chain lengths ranging from 10 (C10) to 35 (C35) carbon atoms (Table 3). Three distinct patterns in chain length distributions were observed (Fig. 5). M2 and M5 both contain only one large peak centered at C25-26, while M3 shows two distinct peaks: one larger peak centered at C25-26 and a smaller peak centered at C17. M1, M6, and M7 contain two peaks, similar to M3; however, these peaks are “connected” by intermediate chain lengths rather than showing two distinct peaks. Degradation of OM may explain the overlap in these distinct peaks, resulting in wider peaks and shorter chain lengths as carbon atoms are cleaved by demethylation (Fig. 6).
Relative abundance distribution of n-alkanes of each sample. Two trends are observed below. The first trend shown in M1, M2, M5 has a large single peak centered around chain length 25, indicating organic material with a terrigenous source. The other trend shown in M3, M6, and M7 consists of two peaks, a large peak centered around chain length 25 indicative of terrigenous organic material and a smaller, minor peak centered around chain length 15, 16, and 17 indicating marine sourced organic material
Despite the differences in lipid concentrations and n-alkane peak distributions, biomarker proxies varied little among samples (Table 4). ACL values were ~ 25 for each sample, ranging from 24.64 (M6) to 26.71 (M5). ATRs ranged from 0.62 (M6) to 0.86 (M5), and CPI values ranged from 0.99 (M5) to 1.04 (M4).
Cerium anomalies ranged from − 0.01 to − 0.14, showing spatial variations in anoxia across New York (Fig. 7, Table 5). Spatial trends were not observed for anoxia across New York State however.
Nickel concentrations ranged from 13 to 297 (mg/kg). A relationship was observed between Nickel and TOM (Fig. 8), with an R2 of 0.945.
The relationship between Ni and TOM is significant and suggests that algae is a probable source for the organic matter in the shale (Lewan and Maynard, 1982). An R2 of 0.9454 was determined for the line of best fit describing the relationship between Ni and TOM
Discussion
The relatively constant ACL values of the-n-alkanes across the Marcellus transect in New York State (~ 25, Table 4) suggest that the majority of the OM is terrestrial in origin (Bianchi and Canuel 2011). A plot of the relative abundance of each n-alkane in the samples (Fig. 5) shows that samples have a bimodal distribution in chain lengths, with a second smaller peak centered around C14 to C18 in M1, M6, M3 and M7. This would suggest that the organic material within these samples is a combination of marine and terrestrial sourced organic material (Bianchi and Canuel 2011). The ATR ratios were all less than one (Table 4), which supports that there is a greater component of terrigenous material that is making up the OM in M1, M6, M3, and M7. Simoneit (1977) observed similar results, where some samples contained bimodal peaks where terrestrial sourced material was the main component of organic material with a minor component of marine material.
The CPI values in every sample were close to 1 (Table 4), which suggests two possibilities. These low values may reflect a mature source rock with petroleum production potential (Bianchi and Canuel 2011). This would place our samples in the late oil window to wet gas window and agrees with the expectation by Bruner and Smosna (2011) for the Marcellus in Central and Western New York. The second possibility is that the terrestrial organic material has been thermally matured to a CPI of one (El Nemr et al. 2016). n-Alkanes can breakdown through demethylation, a process where a terminal methyl group is cleaved and replaced with a hydrogen atom. Demethylation of n-alkanes occurs naturally through microbial degradation and can affect the organic chemistry of oil and gas (Guo et al. 2020). The loss of methyl groups results in artificially low CPI values that do not accurately represent the initial organic material composition. The distribution of chain lengths in our samples, however, agrees with the first possibility, and the relative abundances of n-alkanes in M3 best demonstrate this. M3 has two distinct peaks centered around chain lengths 17–18 and 25–26, clearly showing two different sources of organic material. These two peaks would not be as distinct if the organic material was degraded.
The smaller peaks consisting of shorter n-alkane chain lengths in samples M1, M3, M6 and M7 indicate that the source of the marine component of the hydrocarbons is largely phytoplankton and algal (Sikes et al. 2009). While phytoplankton and algae are sources of high hydrocarbon potential, the sample’s potential would be dominantly controlled by the overwhelming amount of terrigenous organic material. While type II kerogen (mixture of marine and terrigenous organic material) has a higher hydrocarbon potential than type III kerogen (solely terrigenous organic material), it is unknown if the amount of marine organic material present is sufficient to have a positive impact on gas potential.
The source of the terrestrial component of the hydrocarbons for all samples was determined to be terrigenous plants because of the abundance of longer n-alkane chains within the samples (Bianchi and Canuel 2011). The terrigenous organic material, sourced from eastern New York, was transported along the Acadian mountains–Catskill delta sediment route. Terrigenous plants were also determined to be the source of the terrestrial OM of the Cretaceous black shales from the eastern edge of Cape Verde Basin analyzed in Simoneit (1997) which had similar chain lengths in their study.
The three n-alkane proxies do not indicate any large variations in the sources or maturity of the shale OM. While there are differences in peak distributions between the samples, the differences in marine peak size may not be enough to change the proxy values due to a much larger terrigenous peak. A spatial trend in percent total lipids with an R2 of 0.5159 is observed, where the percent total lipids increase toward the east (Fig. 4). This trend would support what is being observed in the source of the organic material. Since the source of sediment is coming from the eroding Acadian Mountains, it would be expected that higher amounts of terrigenous organic material would be deposited closer toward the Acadian Mountains in the East. A larger sample size is needed to further investigate this possible trend.
The cerium anomalies for most samples were negative, indicating that the basin was under anoxic conditions during deposition (Quinby-Hunt et al. 1989). However, M3 and M6 are considered to be deposited during suboxic conditions because the anomalies are close to zero. This is consistent with Werne et al. (2002), who determined that the lower Oatka Creek Formation of the Marcellus was deposited under anoxic conditions. However, there is no clear trend in anoxic values across the basin. This could be because of varying depth within the basin, with areas that were shallower and thus suboxic compared to deeper anoxic portions of the basin. Further 3D stratigraphic controls are needed to better understand anoxic conditions across the basin.
The TOM also did not show any spatial trends across New York State. A correlation between nickel and TOM was found with an R2 value of 0.945. This indicates that algae were the most probable source of the marine sourced organic material, which supports the results from the lipid biomarkers. (Lewan and Maynard 1982).
Based on the results of the inorganic and organic analysis, it was determined that the kerogen type of the Marcellus Formation in New York State was type III (Table 1). This is because the source of the organic material is primarily terrigenous plants. Type III kerogen is consistent with the results of Nyahay et al. (2007), who also studied the Marcellus Formation in New York State. In other locations of the Appalachian basin, the kerogen type of the Marcellus has been determined to be type II or type III (Agrawal and Sharma, May 2018, Obermajer et al. 1997, Pernia et al. 2015). There is also a smaller component of marine input which is phytoplankton and algal in origin. The CPI suggests that the hydrocarbons within the samples are mature enough to produce petroleum along the sampled transect.
Conclusion
Overall, there were no major spatial variations when looking at the organic material of the Marcellus Formation. There was a slight apparent trend where the total amount of lipids was shown to increase as you go east across New York State (Fig. 4). The ACL for all samples was calculated to be ~ 25, and the ATR for all samples was calculated to be less than 1. These values indicate that the organic material within the Marcellus in New York State is primarily terrestrial in origin. There is also a marine sourced component contributing to the organic material within the samples (Fig. 5). Based on the ACL and the other longer chain lengths within the samples, it was determined that the source of the terrestrial organic material was terrigenous plants, and the source of the marine organic material was determined to be phytoplankton and algal because of the abundance of shorter chain lengths around 15–17. The calculated CPI values within the samples were around one and indicates that the samples have been matured enough indicating that the Marcellus Formation in New York should produce petroleum.
The calculated cerium anomalies for each sample were all negative, indicating that the basin was anoxic during deposition. Two samples, however, M3 and M6, were close to zero and can be considered suboxic (Fig. 7). A correlation between nickel and TOM was observed and indicates that the source of the marine input of the organic material is algal. This supports what was observed from the lipid biomarker analysis. TOM and anoxia did not show any spatial trends across the basin. This could be because there was varying local depth within the basin across New York causing anoxia and TOM to vary across the entire basin. Overall, the Marcellus Formation in New York State was determined to contain type III kerogen and could prove to be an economic asset to New York in the future.
Data and material availability
Data transparency.
Code availability
Software application or custom code.
References
Agrawal V, Sharma S (2018) Testing utility of organogeochemical proxies to assess sources of organic matter, paleoredox conditions and thermal maturity in mature Marcellus Shale. Frontiers in Energy Research 6:42
Agrawal V, Sharma S (2018) Molecular characterization of kerogen and its implications for determining hydrocarbon potential, organic matter sources and thermal maturity in Marcellus Shale. Fuel 228:429–437
Ayyıldız T, Varol B, Karakaş Z, Sözeri K (2019) Basic geochemical characteristics of lacustrine rocks in the Neogene Kağızman-Tuzluca Basin, Northeastern Turkey. Journal of Petroleum Exploration and Production Technology 9(1):141–157
Bianchi TS, Canuel EA (2011) Chemical biomarkers in aquatic ecosystems. Princeton University Press
Bianchi TS, Schreiner KM, Smith RW, Burdige DJ, Woodard S, Conley DJ (2016) Redox effects on organic matter storage in coastal sediments during the Holocene: A biomarker/proxy perspective. Annu Rev Earth Planet Sci 44:295–319
Bligh EG, Dyer WJ (1959) A rapid method of total lipid extraction and purification. Can J Biochem Physiol 37(8):911–917
Bray EE, Evans ED (1961) Distribution of n-paraffins as a clue to recognition of source beds. Geochim Cosmochim Acta 22(1):2–15
Bruner KR, Smosna R (2011) A comparative study of the Mississippian Barnett shale, Fort Worth Basin, and Devonian Marcellus shale. Appalachian Basin 106
Canuel EA, Martens CS (1993) Seasonal variations in the sources and alteration of organic matter associated with recently-deposited sediments. Org Geochem 20(5):563–577
Chen R, Sharma S (2016) Role of alternating redox conditions in the formation of organic-rich interval in the Middle Devonian Marcellus Shale, Appalachian Basin, USA. Palaeogeogr Palaeoclimatol Palaeoecol 446:85–97
Didyk BM, Simoneit BRT, Brassell ST, Eglinton G (1978) Organic geochemical indicators of palaeoenvironmental conditions of sedimentation. Nature 272(5650):216
Driese SG, Mora CI, Elick JM (1997) Morphology and taphonomy of root and stump casts of the earliest trees (Middle to Late Devonian), Pennsylvania and New York, USA. Palaios 12(6):524–537
Eglinton G, Hamilton RJ (1967) Leaf epicuticular waxes. Science 156(3780):1322–1335
El Nemr A, Moneer AA, Ragab S, El Sikaily A (2016) Distribution and sources of n-alkanes and polycyclic aromatic hydrocarbons in shellfish of the Egyptian Red Sea coast. The Egyptian Journal of Aquatic Research 42(2):121–131
Gao Y, Wen Z, Xu Y, Song H, Li W, Yu Y, Ke C (2020) Geochemical characteristics of the Chang7 organic-rich fine-grained sedimentary rocks and its relationship with the tight oil in Longdong area, Northwest China. J Pet Explor Prod Technol 1–14
Grice K, Eiserbeck C (2013) The analysis and application of biomarkers. In Treatise on geochemistry. Elsevier Science, p 47–78
Griffing DH, Ver Straeten CA (1991) Stratigraphy and depositional environments of the lower part of the Marcellus Formation (Middle Devonian) in eastern New York State. In New York State Geological Association 63rd Annual Meeting, SUNY Oneonta, p 205–234
Guo H, Tang S, Xie S, Wang P, Huang C, Geng X, Fang J (2020) The oil removal and the characteristics of changes in the composition of bacteria based on the oily sludge bioelectrochemical system. Sci Rep 10(1):1–10
Jeng WL (2006) Higher plant n-alkane average chain length as an indicator of petrogenic hydrocarbon contamination in marine sediments. Mar Chem 102(3–4):242–251
Kargbo DM, Wilhelm RG, Campbell DJ (2010) Natural gas plays in the Marcellus Shale: Challenges and potential opportunities. Environ Sci Technol 44(15):5679–5684
Kennicutt MC II, Barker C, Brooks JM, DeFreitas DA, Zhu GH (1987) Selected organic matter source indicators in the Orinoco Nile and Changjiang Deltas. Organic Geochemistry 11(1):41–51
Kostecki P, Morrison R, Dragun J (2005) HYDROCARBONS. Encyclopedia of Soils in the Environment, 217–226
Lash GG, Engelder T (2011) Thickness trends and sequence stratigraphy of the Middle Devonian Marcellus Formation, Appalachian Basin: Implications for Acadian foreland basin evolution. AAPG Bull 95(1):61–103
Lewan MD, Maynard JB (1982) Factors controlling enrichment of vanadium and nickel in the bitumen of organic sedimentary rocks. Geochim Cosmochim Acta 46(12):2547–2560
Mani D, Kalpana MS, Patil DJ, Dayal AM (2017) Organic Matter in Gas Shales: Origin, Evolution, and Characterization. In Shale Gas, p 25–54
Mazzullo SJ (1973) Deltaic depositional environments in the Hamilton Group (Middle Devonian, southeastern New York state). J Sediment Res 43(4):1061–1071
Meyers PA (1997) Organic geochemical proxies of paleoceanographic, paleolimnologic, and paleoclimatic processes. Org Geochem 27(5–6):213–250
Meyers PA, Ishiwatari R (1993) The early diagenesis of organic matter in lacustrine sediments. In Organic geochemistry. Springer, Boston, MA. p 185–209
Miceli Romero A, Philp RP (2012) Organic geochemistry of the Woodford Shale, southeastern Oklahoma: How variable can shales be? AAPG Bull 96(3):493–517
Mißbach H, Duda JP, Lünsdorf NK, Schmidt BC, Thiel V (2016) Testing the preservation of biomarkers during experimental maturation of an immature kerogen. Int J Astrobiol 15(3):165
Nyahay RE, Leone J, Smith LB, Martin JP, Jarvie DJ (2007) Update on Regional Assessment of Gas Potential in the Devonian Marcellus and Ordovician Utica Shales of New York, Thirty-Sixth Meeting of the Eastern Section. American Association of Petroleum Geologists, Lexington, KY.
Obermajer M, Fowler MG, Goodarzi F, Snowdon LR (1997) Organic petrology and organic geochemistry of Devonian black shales in southwestern Ontario Canada. Organic Geochemistry 26(3–4):229–246
Pernia D, Bissada KA, Curiale J (2015) Kerogen based characterization of major gas shales: Effects of kerogen fractionation. Org Geochem 78:52–61
Peters KE, Walters CC, Moldowan JM (2005) The biomarker guide, I: Biomarkers and isotopes in the environment and human history, 2nd edn. Cambridge University Press. Cambridge, UK
Popova O, Frye E, Panarelli E (2015).Updated geologic maps provide greater detail for Marcellus formation. https://www.eia.gov/todayinenergy/detail.php?id=20612#. Retrieved 19 July 2020
Poynter J, Eglinton G (1990) Molecular composition of three sediments from hole 717c: The Bengal fan. In Proceedings of the Ocean Drilling Program: Scientific results, vol 116, p 155–161
Qu J, Ding X, Zha M, Chen H, Gao C, Wang Z (2017) Geochemical characterization of Lucaogou Formation and its correlation of tight oil accumulation in Jimsar Sag of Junggar Basin, Northwestern China. Journal of Petroleum Exploration and Production Technology 7(3):699–706
Quinby-Hunt MS, Wilde P, Orth CJ, Berry WBN (1989) Elemental geochemistry of black shales—statistical comparison of low-calcific shales with other shales. Metalliferous Black Shales and Related Ore Deposits. US Geol Surv Circ 1037:8–15
Sikes EL, Uhle ME, Nodder SD, Howard ME (2009) Sources of organic matter in a coastal marine environment: evidence from n-alkanes and their δ13C distributions in the Hauraki Gulf New Zealand. Marine Chemistry 113(3–4):149–163
Simoneit BRT (1977) Leg 41 sediment lipids—Search for eolian organic matter in Recent samples and examination of a black shale. Init Rep Deep Sea Drilling Proj 41:855–858
Smith LB, Schieber J, Wilson RD (2019) Shallow-water onlap model for the deposition of Devonian black shales in New York, USA. Geology 47(3):279–283
Theresa NA, Godwin DA, Charles CC, Oguejiofo TU (2018) Comparative assessment of carbon preference index and degree of waxiness in different crude oils as indicator of maturity and organic matter input: a case study of Ebocha and Kwali crude oil Niger Delta, Nigeria. Recent Adv Petrochem Sci 4(1):555630
Tissot BP, Pelet R, Ungerer PH (1987) Thermal history of sedimentary basins, maturation indices, and kinetics of oil and gas generation. AAPG Bull 71(12):1445–1466
Werne JP, Sageman BB, Lyons TW, Hollander DJ (2002) An integrated assessment of a “type euxinic” deposit: evidence for multiple controls on black shale deposition in the Middle Devonian Oatka Creek Formation. Am J Sci 302(2):110–143
Xie YL, Dearing JA, Blomendal I (2000) Organic matter content of street dust in Liverpool, UK and its association with dust magnetic properties. Atmos Environ 34:269–275
Funding
Research for this project was partially supported by Global Aquatic Research LLC, and the Student Undergraduate Research Program at SUNY Brockport.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
The author declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Blocho, R.M., Smith, R.W. & Noll, M.R. Analyses of depositional environments of the Marcellus formation in New York using biomarker and trace metal proxies. J Petrol Explor Prod Technol 11, 3163–3175 (2021). https://doi.org/10.1007/s13202-021-01237-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13202-021-01237-8