Abstract
A novel enhanced oil recovery (EOR) method based on polymer gel-assisted carbon dioxide (CO2) huff and puff was developed aiming to improve the development effect of heavy oil reservoirs with high water cut. The polymer gel prepared using partially hydrolyzed polyacrylamide (HPAM), hexamethylenetetramine, phenol, resorcinol, oxalic acid, and thiocarbamide as raw materials had a special network structure to overcome the challenge of significant heterogeneity in heavy oil reservoirs. The strength of polymer gel reached the maximum value 20,000 mPa·s within 22 h. The temperature resistance and salt resistance of polymer gel directly determined the plugging effect. The polymer gel was placed for 190 days at 85 ℃, and its apparent viscosity retention rate was 66.4%. The salt resistance experiments showed that the apparent viscosity retention rate of this polymer gel at 1.8 wt % NaCl, 0.045 wt % CaCl2, 0.045 wt % MgCl2 was 71.3%, 78.5%, 71.4%, respectively. Huff and puff experiments confirmed that this method could be used to increase the sweep volume and improve the oil displacement efficiency of heavy oil reservoirs with high water cut. Furthermore, the EOR of this method was better than that of water huff and puff, polymer gel huff and puff or CO2 huff and puff.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
It is well known that the geological reserves of heavy oil are much higher than that of conventional crude oil (Huang et al. 2018; Guo et al. 2016). With the decreasing of conventional crude oil recoverable reserves, the position of heavy oil resources in energy structure is more and more important (Amirian et al. 2018; Cao and Gu 2013).
At present, thermal recovery technology (such as steam-assisted gravity drainage, steam huff and puff, steam flooding, burning oil layer, and hot water flooding) is used in the world to develop the heavy oil reservoir with a depth less than 1000 m and a thickness more than 10 m (Lyu et al. 2018; Butler et al. 2010; Sherratt et al. 2018; Ahmadi et al. 2014; Xu et al. 2013). Unfortunately, due to the reservoir heterogeneity, the displacing medium (steam, hot water, etc.) will flow along the high-capacity channel during the thermal recovery process, and the sweep efficiency of the displacing medium is relatively low (Ravalec et al. 2009; Kharrat et al. 2011; Ali et al. 2012). As a result, the water cut of heavy oil reservoir will reach 90% rapidly, and the recovery of thermal recovery technology is usually less than 20% OOIP (original oil in place) (Salmo et al. 2017; Taborda et al. 2016; Riazi and Golkari 2016; Mayorquin-Ruiz and Babadagli 2016). Moreover, thermal recovery technology generally requires high temperature (at least 95 ℃), and it is very difficult to increase sweep efficiency using chemical methods under this conditions. Therefore, the effect of thermal recovery technology in high water cut heavy oil reservoir is generally not satisfactory (Salmo et al. 2017; Riazi and Golkari 2016).
In addition to the thermal recovery technology, the chemical cold recovery technology, such as polymer flooding, surfactant flooding, alkaline water flooding, carbon dioxide (CO2) flooding, and chemical huff and puff, is mainly used for the exploitation of the heavy oil reservoir with a depth more than 1000 m or the marginal heavy oil reservoir with small reserves (Guo et al. 2013a; Sedaghat et al. 2013; Zhang et al. 2016; Jamaloei et al. 2016). Although chemical cold recovery technology can effectively reduce the viscosity of crude oil and interphase interface tension, the chemical agents are easy to form adsorption on the surface of the rock or dissolve in formation water, resulting in the waste of chemical agents and high cost (Guo et al. 2013b; Pei et al. 2014; Coskuner et al. 2015; Mohsenzadeh et al. 2015). At the same time, the primary and secondary high capacity channel will greatly reduce the application effect of chemical cold recovery technology (Ravalec et al. 2009; Kharrat et al. 2011; Ali et al. 2012). Similar to thermal recovery technology, the water cut of heavy oil reservoirs increases rapidly using cold recovery technology. However, there are very few methods to improve the recovery of heavy oil reservoirs with high water cut (Yang et al. 2018; Fang et al. 2018; Liu et al. 2017; Alemu et al. 2013; Wang and Gu 2011; Zhao et al. 2011). With the deepening of the world’s oil production, how to improve the recovery of heavy oil with high water cut economically and effectively is a challenge for petroleum workers.
Polymer gel plays a good role in preventing gas channeling in CO2 flooding (Secaeddin et al. 2012). Keep in mind the requirement of enhanced oil recovery (EOR) technology, herein, a novel EOR method based on polymer gel-assisted CO2 huff and puff was proposed aiming to develop heavy oil reservoirs with high water cut economically and effectively. The main ideas of this method are as follows: (1) polymer gel is used to block the high capacity channel, prevent CO2 from channeling along the high capacity channel and increase sweep efficiency of CO2. (2) CO2 is injected to improve the flow performance of heavy oil. After dissolved in crude oil, CO2 can reduce the viscosity of heavy oil and interfacial tension of heavy oil/water. (3) Shut the well to make CO2 and heavy oil fully function. (4) Open-well production.
In this paper, a new method to enhance oil recovery for high water cut heavy oil reservoirs after thermal recovery or cold recovery was provided, especially for the heavy oil reservoirs with a depth more than 1000 m and the marginal heavy oil reservoir with small reserves.
Experimental section
Materials
Partially hydrolyzed polyacrylamide (HPAM, degree of hydrolysis: 20%, viscosity molecular weight: 20 × 106) was purchased from Sichuan Guangya Polymer Chemical Co., Ltd. Hexamethylenetetramine (C6H12N4, AR), phenol (C6H6O, AR), resorcinol (C6H6O2, AR), oxalic acid (C2H2O4, AR), thiocarbamide (CH4N2S, AR), sodium chloride (NaCl, AR), potassium chloride (KCl, AR), calcium chloride anhydrous (CaCl2, AR), magnesium chloride hexahydrate (MgCl2, AR), sodium bicarbonate (NaHCO3, AR), sodium sulfate (Na2SO4, AR), and kerosene were obtained from Chengdu Kelong Chemical Reagent Plant. All chemicals and reagents were used as received without any further purification. CO2 with purity of 99.9% was purchased from Tangshan Sihai Gas Company. The injected water and formation water were taken from Jidong Oilfield. Their ionic composition is shown in Table 1.
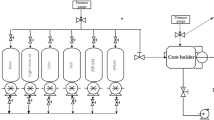
Crude oil was obtained from Jidong Oilfield. The density and apparent viscosity of the crude oil on the ground were 0.956 g/cm3 and 1089.9 mPa·s at 25 ℃, respectively. Oil sand was taken from Jidong Oilfield. The crude oil on the oil sand surface was cleaned by kerosene and hot water in turn and then dried for 24 h at 100 ℃. The length and diameter of sand packed model (Fig. 1) were 30 cm and 2.5 cm, respectively.
Preparation of polymer gel
In a 1000-ml beaker, 400 g injected water and 1.8 g HPAM were added, and the polymer solution was prepared after 2 h of agitation at room temperature. A total of 200 g injected water, 0.48 g hexamethylenetetramine, 0.48 g phenol, 0.09 g resorcinol, 0.3 g oxalic acid, and 0.06 g thiocarbamide were added to a 500-ml beaker, respectively. The mixed solution was stirred for 20 min at room temperature, and the cross-linker solution was obtained. Then the cross-linker solution was added to the polymer solution and stirred for 5 min to prepare the mixture of polymer solution and cross-linker solution (MPSC).
Gelation time and strength
The glass bottle equipped with MPSC was placed in the thermostat at a temperature of 65 ℃, and the apparent viscosity of polymer gel was regularly measured with a Brookfield DV3T viscometer at 65 ℃.
Temperature resistance
MPSC prepared using the injected water was packed in three glass bottles, and these glass bottles were put into the thermostats with different temperatures (65 ℃, 75 ℃, 85 ℃). The apparent viscosity of polymer gel small sample was regularly measured with a Brookfield DV3T viscometer at 65 ℃.
Salt resistance
The salt resistance of MPSC was investigated by NaCl, CaCl2, and MgCl2 with different concentrations, respectively. The apparent viscosity of MPSC after gelling was measured with a Brookfield DV3T viscometer at 65 ℃.
Evaluation experiment of profile improvement performance
Double-tube experiments were carried out to study the profile improvement ability of polymer gel at 65 ℃. After being cleaned and dried, the oil sand was used to fill the sand packed model, and the filling pressure was 10 MPa. The high-permeability sand packed model and the low-permeability sand packed model were prepared using 40–80 mesh oil sand and 100–140 mesh oil sand, respectively. The experimental instruments were installed as shown in Fig. 2. In the process of experiments, the back pressure was 12 MPa.
The sand packed models were saturated with the formation water at 1.0 ml/min until injection pressure was stable. 0.3 PV (pore volume) MPSC was injected into the sand packed models at 0.2 ml/min. At 48 h later, the injected water was injected into the sand packed models at 1 ml/min until pressure was stable. The water production of the sand packed models was measured.
The profile improvement rate of polymer gel was calculated with the following equation:
where f is the profile improvement rate of polymer gel, %; Qhb is liquid flow velocity of the high-permeability sand packed model before polymer gel plugging, ml/min; Qha is liquid flow velocity of the high-permeability sand packed model after polymer gel plugging, ml/min; Qlb is liquid flow velocity of the low-permeability sand packed model before polymer gel plugging, ml/min; Qla is liquid flow velocity of the low-permeability sand packed model after polymer gel plugging, ml/min.
Polymer gel-assisted CO2 huff and puff experiment
The huff and puff experiments were used to study the EOR effect of polymer-assisted CO2 huff and puff at 65 ℃. According to Fig. 2, the experimental devices were connected. All experiments were carried out under the 12 MPa back pressure. The formation water was injected into the sand packed models at 1 ml/min until pressure was stable. The sand-packed models were saturated with the crude oil at 0.2 ml/min to obtain bound water saturation, and then the injected water was used to displace the crude oil at 1 ml/min until the water cut reached 99%.
According to Fig. 3, the experimental devices were prepared. 0.1 PV MPSC was injected into the sand packed models at 0.2 ml/min. 48 h later, 0.2 PV CO2 (temperature 65 ℃, pressure 12 MPa) was injected into the sand packed models at 0.2 ml/min. Ten days later, the outlet valves were opened, and the oil production of the sand packed models was measured.
The EOR of the polymer gel-assisted CO2 huff and puff was calculated with the following equation:
where EOR is enhanced oil recovery of the polymer gel-assisted CO2 huff and puff, %; E1 is the oil production of the whole experiment process, ml; E2 is the oil production of water flooding stage, ml; E0 is the amount of crude oil in the sand packed models, ml.
According to the above experimental method, EOR of water huff and puff, CO2 huff and puff, and polymer gel huff and puff was determined under the same conditions. The amount of water, CO2, and MPSC was 0.3 PV.
Results and discussion
Gelation time and strength
The apparent viscosity–time curve of polymer gel is presented in Fig. 4. When MPSC was put into the thermostat at a temperature of 65 ℃, its apparent viscosity increased rapidly. After 22 h, the apparent viscosity of polymer gel reached 20,000 mPa·s. Subsequently, the apparent viscosity of polymer gel fluctuated at around 20,000 mPa·s. It could be found that the gelation time and strength of polymer gel were 22 h and 20,000 mPa·s, respectively.
Temperature resistance
The influence of temperature on the apparent viscosity of polymer gel was studied. The results are shown in Fig. 5. The apparent viscosity of polymer gel decreased slowly with the increase in storage time at different temperatures. After 190 days, the apparent viscosity of polymer gel at 65 ℃, 75 ℃, and 85 ℃ was 17,450 mPa·s, 16,060 mPa·s, and 13,280 mPa·s, and the apparent viscosity retention rate of polymer gel was 87.3%, 80.3%, and 66.4%, respectively. The colloidal morphology of polymer gel is shown in Fig. 6. We could easily find that the colloid was less affected by temperature, and the gel breaking phenomenon is not observed in Fig. 6. The above experiments proved that the temperature resistance of polymer gel basically met most heavy oil reservoir requirements, because the temperature of these heavy oil reservoir was usually below 75 ℃ (Sun et al. 2017; Xie et al. 2017; Zuloaga et al. 2017).
Salt resistance
The influence of NaCl on apparent viscosity of polymer gel is shown in Fig. 7. The influence of CaCl2 or MgCl2 on apparent viscosity of polymer gel is shown in Fig. 8. It could be found that the decrease in apparent viscosity of polymer gel was smaller when the concentration of NaCl exceeded 1.0 wt % or the concentration of two valence salt (CaCl2 or MgCl2) exceeded 0.03 wt %. This phenomenon could be well explained by the polyelectrolyte behavior of the polymer in brine-free water (Liu et al. 2013). Because the concentration of cations was increased in the solution, the stretched polyelectrolyte chain started shrinking due to a reduction in intra-anionic electrostatic repulsion. When the cations concentration reached the required amount of complete shrinking, the apparent viscosity would no longer decreased with the increase of salt concentration (Liu et al. 2012; Ye et al. 2013). The apparent viscosity retention rate of this polymer gel at 1.8 wt % NaCl, 0.045 wt % CaCl2, and 0.045 wt % MgCl2 was 71.3%, 78.5%, 71.4%, respectively. The buried depth of conventional heavy oil reservoirs was less than 1000 m. The contents of NaCl, CaCl2, and MgCl2 in formation water were usually lower than 1.8 wt %, 0.045 wt %, and 0.045 wt %, respectively. Therefore, the salt resistance of MPSC could meet the requirement of plugging the high capacity channel in heavy oil reservoirs.
Profile improvement ability of polymer gel
The parameters and results of the double tube experiments are shown in Table 2. Before the polymer gel plugging, the liquid flow velocity of the high-permeability sand packed model and the low-permeability sand packed model were 0.83 ml/min and 0.17 ml/min, respectively. During water injection process after polymer gel plugging, the liquid flow velocity of the high-permeability sand packed model was reduced to 0.32 ml/min. The liquid flow velocity of the low-permeability sand packed model was increased to 0.68 ml/min correspondingly. Profile improvement rate of the polymer gel was 89.6% under the above experimental conditions. The experimental results confirmed that the water injection profile was improved after polymer gel plugging.
The sand in the sand packed model was cooled by liquid nitrogen and then vacuumed. Scanning electron microscopy (SEM) was used to observe the plugging of polymer gel at room temperature. As shown in Fig. 9c, we could clearly found that there existed space network structures of the polymer gel after being cooled and dried in the pore throat of the sand. The results indicated that the high-permeability sand packed model was blocked by polymer gel. Therefore, its permeability was greatly reduced after the polymer gel was injected (Lai et al. 2013). In addition, due to the presence of electrostatic and hydrogen bonds, the polymer gel would be adsorbed on the surface of the sand, which helped to improve the blocking strength and anti-erosion property of polymer gel (Xu et al. 2015; Alcázar-Vara et al. 2015; Cheraghian et al. 2017; Pang et al. 2015). As shown in Fig. 9b, d, on the contrary, polymer gel had little effect on the pore throat of the sand in low-permeability sand packed model, because MPSC mainly entered high-permeability sand packed model during injection process.
SEM images of porous medium: a high-permeability sand packed model before plugging, at 100 μm, 400 × ; b low-permeability sand packed model before plugging, at 100 μm, 400 × ; c high-permeability sand packed model after plugging, at 100 μm, 400 × ; d low-permeability sand packed model after plugging, at 100 μm, 400 ×
EOR of the polymer gel-assisted CO2 huff and puff
Huff and puff experiments were carried out to evaluate the EOR effect of several different huff and puff methods under the same experimental conditions. The parameters of the sand packed models and experimental results are shown in Table 3. In the four huff and puff methods, the polymer gel-assisted CO2 huff and puff had the largest EOR, the second was the CO2 huff and puff, and the water huff and puff was the least.
As shown in Table 3, most of the oil production came from the high-permeability sand packed models (3#, 5#, 7#, 9#) due to smaller flow resistance to crude oil in the water flooding stage. For water huff and puff and CO2 huff and puff, as the high-capacity channel being not blocked, the injected huff and puff media mainly entered the high-permeability sand packed models in the huff stage, and most of these media could be taken out during the puff stage. Because the remaining oil of the high-permeability sand packed models was less, the EOR of these two methods was relatively low. Compared with water huff and puff, the EOR of CO2 huff and puff was higher, because CO2 could dissolve in crude oil and reduce oil/water interfacial tension. At the same time, CO2 could reduce the viscosity of crude oil (Mao et al. 2018; Li et al. 2018; Ren et al. 2017; Fang et al. 2017).
For polymer gel-assisted CO2 huff and puff, the high-permeability sand packed model would be blocked by polymer gel, and CO2 would enter the low-permeability sand packed model with higher oil saturation. Then the crude oil would be produced from the low-permeability sand packed model. Compared with polymer gel huff and puff, polymer gel-assisted CO2 huff and puff could not only expand the sweep volume, but also improve the oil displacement efficiency, so it had a higher EOR (11.30% vs. 4.88%).
Conclusions
A novel EOR method base on polymer gel-assisted CO2 huff and puff was put forward aiming to improve the development effect of heavy oil reservoirs with high water cut. A polymer gel was prepared using HPAM, hexamethylenetetramine, phenol, resorcinol, oxalic acid, and thiocarbamide as raw materials, and its heat resistance and salt tolerance could meet the requirement of plugging high capacity channel for heavy oil reservoirs. The experimental results confirmed that this method could be used to increase the sweep volume and improve the oil displacement efficiency of heavy oil reservoirs. The EOR of this method was better than that of water huff and puff, polymer gel huff and puff or CO2 huff and puff.
References
Ahmadi MA, Zendehboudi S, Bahadori A, James L, Lohi A, Elkamel A, Chatzis I (2014) Recovery rate of vapor extraction in heavy oil reservoirs-experimental, statistical, and modeling studies. Ind Eng Chem Res 53:16091–16106
Alcázar-Vara LA, Zamudio-Rivera LS, Buenrostro-González E (2015) Multifunctional evaluation of a new supramolecular complex in enhanced oil recovery (EOR), removal/control of organic damage and heavy crude oil viscosity reduction. Ind Eng Chem Res 54:7766–7776
Alemu BL, Aker E, Soldal M, Johnsen Ø, Aagaard P (2013) Effect of sub-core scale heterogeneities on acoustic and electrical properties of a reservoir rock: a CO2 flooding experiment of brine saturated sandstone in a computed tomography scanner. Geophys Prospect 61(1):235–250
Ali F, Hamed A, Nawi DM, Hussin YM, Soheil N (2012) Impact of reservoir heterogeneity on steam assisted gravity drainage in heavy oil fractured reservoirs. Energ Explor Exploit 30:553–566
Amirian E, Dejam M, Chen Z (2018) Performance forecasting for polymer flooding in heavy oil reservoirs. Fuel 216:83–100
Butler RM, Mcnab GS, Lo HY (2010) Theoretical studies on the gravity drainage of heavy oil during in-situ steam heating. Can J Chem Eng 59:455–460
Cao M, Gu Y (2013) Oil recovery mechanisms and asphaltene precipitation phenomenon in immiscible and miscible CO2 flooding processes. Fuel 109:157–166
Cheraghian G, Kiani S, Nassar NN, Alexander S, Barron AR (2017) Silica nanoparticle enhancement in the efficiency of surfactant flooding of heavy oil in a glass micromodel. Ind Eng Chem Res 56:8528–8534
Coskuner G, Naderi K, Babadagli T (2015) An enhanced oil recovery technology as a follow up to cold heavy oil production with sand. J Petrol Sci Eng 133:475–482
Fang TM, Wang MH, Wang C, Liu B, Shen Y, Dai CL, Zhang J (2017) Oil detachment mechanism in CO2 flooding from silica surface: molecular dynamics simulation. Chem Eng Sci 164:17–22
Fang T, Wang M, Li J, Liu B, Shen Y, Yan Y, Zhang J (2018) Study on the asphaltene precipitation in CO2 flooding: a perspective from molecular dynamics simulation. Ind Eng Chem Res 57:1071–1077
Guo ZQ, Dong MZ, Chen ZX, Yao J (2013a) Dominant scaling groups of polymer flooding for enhanced heavy oil recovery. Ind Eng Chem Res 52:911–921
Guo Z, Dong M, Chen Z, Yao J (2013b) A fast and effective method to evaluate the polymer flooding potential for heavy oil reservoirs in western Canada. J Petrol Sci Eng 112:335–340
Guo K, Li H, Yu Z (2016) In-situ heavy and extra-heavy oil recovery: a review. Fuel 185:886–902
Huang SJ, Cao M, Cheng LS (2018) Experimental study on aquathermolysis of different viscosity heavy oil with superheated steam. Energy Fuels 2018(32):4850–4858
Jamaloei BY, Babolmorad R, Kharrat R (2016) Visualization and analysis of viscous fingering in alcohol-assisted surfactant waterflooding of heavy oil in a two-dimensional sandstone micromodel. Fuel 184:169–179
Kharrat R, Ghazanfari MH, Vossoughi S, Dehghan AA (2011) Quantifying the role of pore geometry and medium heterogeneity on heavy oil recovery during solvent/co-solvent flooding in water-wet systems. J Porous Media 14:363–373
Lai NJ, Qin XP, Ye ZB, Li CX, Chen K, Zhang Y (2013) The study on permeability reduction performance of a hyperbranched polymer in high permeability porous medium. J Petrol Sci Eng 112(3):198–205
Li T, Xu J, Zou R, Feng H, Li L, Wang JY, Stuart MAC, Guo XH (2018) Resin from liaohe heavy oil: molecular structure, aggregation behavior and effect on oil viscosity. Energy Fuels 32:306–313
Liu XJ, Jiang WC, Gou SH, Ye ZB, Xie XD (2012) Synthesis and evaluation of a water-soluble acrylamide binary sulfonates copolymer on MMT crystalline interspace and EOR. J Appl Polym Sci 125(2):1252–1260
Liu XJ, Jiang WC, Gou SH, Ye ZB, Feng MM, Lai NJ, Liang LX (2013) Synthesis and evaluation of novel water-soluble copolymers based on acrylamide and modular β-cyclodextrin. Carbohyd Polym 96(1):47–56
Liu Y, Teng Y, Jiang LL, Zhao JF, Zhang Y, Wang DY, Song YC (2017) Displacement front behavior of near miscible CO2 flooding in decane saturated synthetic sandstone cores revealed by magnetic resonance imaging. Magn Reson Imaging 37:171–178
Lyu X, Liu H, Pang Z, Sun Z (2018) Visualized study of thermochemistry assisted steam flooding to improve oil recovery in heavy oil reservoir with glass micromodels. Fuel 218:118–126
Mao JC, Liu JW, Peng YK, Zhang ZY, Zhao JZ (2018) Quadripolymers as viscosity reducers for heavy oil. Energy Fuels 32:119–124
Mayorquin-Ruiz J, Babadagli T (2016) Low temperature air injection with solvents in heavy-oil containing naturally fractured reservoirs: effects of matrix/fracture properties and temperature on recovery. Fuel 179:376–390
Mohsenzadeh A, Al-Wahaibi Y, Jibril A, Al-Hajri R, Shuwa S (2015) The novel use of deep eutectic solvents for enhancing heavy oil recovery. J Petrol Sci Eng 130:6–15
Pang Z, Liu H, Zhu L (2015) A laboratory study of enhancing heavy oil recovery with steam flooding by adding nitrogen foams. J Petrol Sci Eng 128:184–193
Pei HH, Zhang GC, Ge JJ, Zhang L, Ma MC (2014) Effect of the addition of low molecular weight alcohols on heavy oil recovery during alkaline flooding. Ind Eng Chem Res 53:1301–1307
Ravalec ML, Morlot C, Marmier R, Foulon D (2009) Heterogeneity impact on SAGD process performance in mobile heavy oil reservoirs. Oil Gas Sci Technol 64:469–476
Ren YW, Chen ZJ, Du H, Fang L, Zhang XD (2017) Preparation and evaluation of modified ethylene–vinyl acetate copolymer as pour point depressant and flow improver for Jianghan crude oil. Ind Eng Chem Res 56:11161–11166
Riazi M, Golkari A (2016) The influence of spreading coefficient on carbonated water alternating gas injection in a heavy crude oil. Fuel 178:1–9
Salmo IC, Pettersen Ø, Skauge A (2017) Polymer flooding at adverse mobility ratio-acceleration of oil production by crossflow into water channels. Energy Fuels 31:5948–5958
Secaeddin S, Ulker K, Demet C, Ersan D, Hakki L (2012) A quarter century of progress in the application of CO2 immiscible EOR project in Bati Raman heavy oil field in Turkey. In: SPE Heavy Oil Conference Canada, Calgary, Alberta, Canada, June 2012. SPE 157865
Sedaghat MH, Ghazanfari MH, Masihi M, Rashtchian D (2013) Experimental and numerical investigation of polymer flooding in fractured heavy oil five-spot systems. J Petrol Sci Eng 108:370–382
Sherratt J, Haddad AS, Rafati R (2018) Hot solvent assisted gravity drainage in naturally fractured heavy oil reservoirs: a new model and approach to determine optimal solvent injection temperature. Ind Eng Chem Res 57:3043–3058
Sun SS, Luo YJ, Zhou Y, Xiao M, Zhang ZY, Hou JR, Wei XF, Xu QS, Sha T, Dong H, Song H, Zhang ZZ (2017) Application of Bacillus spp. in pilot test of microbial huff and puff to improve heavy oil recovery. Energy Fuels 31:13724–13732
Taborda EA, Franco CA, Lopera SH, Alvarado V, Cortés FB (2016) Effect of nanoparticles/nanofluids on the rheology of heavy crude oil and its mobility on porous media at reservoir conditions. Fuel 184:222–232
Wang X, Gu Y (2011) Oil recovery and permeability reduction of a tight sandstone reservoir in immiscible and miscible CO2 flooding processes. Ind Eng Chem Res 50(4):2388–2399
Xie Q, Chen YQ, Sari A, Pu WF, Saeedi A, Liao XW (2017) A pH-resolved wettability alteration: implications for CO2-assisted EOR in carbonate reservoirs. Energy Fuels 31:13593–13599
Xu A, Mu L, Fan Z, Wu X, Zhao L, Bo B (2013) Mechanism of heavy oil recovery by cyclic superheated steam stimulation. J Petrol Sci Eng 111:197–207
Xu J, Jiang HJ, Li T, Wei XM, Wang TS, Jing H, Wang WN, Smith AL, Wang J, Zhang R, Xu YS, Li L, Prud’homme RK, Guo XH (2015) Effect of comb-type copolymers with various pendants on flow ability of heavy crude oil. Ind Eng Chem Res 54:5204–5212
Yang F, Chen YQ, Sun GY, Yang S, Li CX, You J, Liu DW (2018) Effects of supercritical CO2 treatment on the stability of water-in-heavy oil emulsion and their mechanisms. Energy Fuels 32:1358–1364
Ye ZB, Gou GJ, Gou SH, Jiang WC, Liu TY (2013) Synthesis and characterization of a water-soluble sulfonates copolymer of acrylamide and n-allylbenzamide as enhanced oil recovery chemical. J Appl Polym Sci 128(3):2003–2011
Zhang HY, Chen GY, Dong MZ, Zhao SQ, Liang ZW (2016) Evaluation of different factors on enhanced oil recovery of heavy oil using different alkali solutions. Energy Fuels 30:3860–3869
Zhao Y, Song Y, Liu Y, Liang H, Dou B (2011) Visualization and measurement of CO2 flooding in porous media using MRI. Ind Eng Chem Res 50(50):4707–4715
Zuloaga P, Yu W, Miao J, Sepehrnoori K (2017) Performance evaluation of CO2 Huff-n-Puff and continuous CO2 injection in tight oil reservoirs. Energy 134:181–192
Funding
This work was funded by the Science and Technology Project of Sichuan University of Science and Engineering (2020RC05).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Lu, H., Wang, Z., Peng, T. et al. A novel method for improving recovery of heavy oil reservoirs with high water cut based on polymer gel-assisted CO2 huff and puff. J Petrol Explor Prod Technol 11, 3533–3541 (2021). https://doi.org/10.1007/s13202-021-01232-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13202-021-01232-z