Abstract
In order to solve the problems due to the thickening of drilling fluids at low temperatures caused by the use of high-molecular-weight polymer coating agents in offshore deep-sea oil and gas drilling, a low-molecular-weight polymer coating agent named PADA was synthesized with acrylamide, methacryloxyethyltrimethyl ammonium chloride, and 2-acrylamido-2-methyl propane sulfonic acid. The PADA polymer was characterized with Fourier transform infrared spectroscopy and gel permeation chromatography. The shale inhibition effects of the PADA polymer and associated mechanisms were investigated by shale recovery and expansion experiments, transmission electron microscopy observation, particle size and zeta potential analysis, and interlayer spacing measurements. In addition, the effects of the coating agent on the filter cakes and the low temperature rheological properties of bentonite mud were also tested, and the polymer biodegradability was evaluated. The results showed that the molecular weight of the PADA polymer was 265,000 D, which was significantly lower than that of the traditional coating agents. The PADA had similar effects as two typical commercial products CAP and HPAM on inhibiting the hydration dispersion of shales and performed better than another product PAM. The inhibition effect was achieved by the polymer absorption onto the clay particles through both hydrogen bonding and the electrostatic interactions. The viscosity of bentonite mud containing PADA was much lower than that of mud with other coating agents at 4 °C, so the serious thickening caused by traditional coating agents at a low temperature could be avoided. In addition, it is relatively easily biodegraded.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The Gulf of Mexico, the North Sea, the West Africa Sea, and the South China Sea are rich in oil and gas (Zhao et al. 2021). The drilling fluid is one of the key technologies in the development of offshore oil and gas drilling. Shales in some offshore formations, especially in deep-sea formations, have strong water sensitivity. Since it is prone to hydration expansion and dispersion on exposure to free water in drilling fluids, it can cause wellbore instability (Liu et al. 2020; Swai 2020; Van Oort 2003). In addition, the temperature around the mudline in deep-sea regions is as low as 2–4 °C, or even below 0 °C. Under this condition, serious thickening of the drilling fluid easily occurs, resulting in high flow resistance and lost circulation (Davison et al. 1999; Herzhaft et al. 2001; Knox et al. 2015). The low temperature poses a significant technical challenge to the drilling fluid.
Years of research and field practices have indicated that the research and development of highly efficient hydration inhibitor along with the use of polymeric coating agent could achieve an effective inhibition of shale hydration (Marin et al. 2009; Swai 2020). The coating agent is usually the high-molecular-weight amide polymer, such as partially hydrolyzed polyacrylamide (HPAM) and polyacrylamide (PAM) (Jain and Mahto 2015; Kadaster et al. 1992; Mehtar et al. 2010; Van Oort 2003). They formed multi-point adsorption on the clay surface of wellbore by hydrogen bonding interaction of –CONH2 and other adsorption groups, bridged the surrounding multiple clay particles together, prevented the dispersion and exfoliation, and maintained the wellbore stability. At the same time, the polymeric coating agent also winded and wrapped up the drilling cuttings in drilling fluid by the effect of adsorption, kept the integrity of drilling cuttings, avoided the hydration dispersion of drilling cuttings to cause the deterioration of drilling fluid performance, and enabled the drilling cuttings to be easily removed by solid control equipment. Therefore, to effectively bridge and wrap the clay particles, the molecular weight of the polymer was usually above 1,500,000 D. However, due to the long polymer molecular chain, it had large contact area with water and resulted in high flow resistance. In addition, the curly molecular chain was prone to form grid structure through intertwining, and thus increased the viscosity of the drilling fluid. Especially in the process of drilling in deep-sea regions, where the temperature near the seabed mudline was as low as 2–4 °C, the polymer molecular chains curled up and entangled with each other under low temperatures. Because of this, the viscosity of drilling fluid substantially increased. Since the safe mud density window between the pore pressure and fracture pressure of shallow formation in deep-sea is very narrow, the substantial increase of rheological parameters of drilling fluid at low temperatures leads to a significant increase in equivalent circulating density (ECD) of the drilling fluid. This will exceed the safe density limit and fracture the formation, resulting in loss of drilling fluid (Davison et al. 1999; Dokhani et al. 2016; Herzhaft et al. 2001; Zamora et al. 2000). In addition, the viscous drilling fluid at low temperatures was prone to adhere to the vibration screen and unable to get through the sieve, thus leading to fluid leakage (Zhao et al. 2017). Since the deep-sea drilling operation has the characteristics of high risk and high investment, these operational problems could seriously affect the efficiency of drilling operation, result in huge economic loss, and increase security risks. The ideal deep-sea drilling fluid should have excellent inhibition ability of clay hydration dispersion and rheological properties at a low temperature. Therefore, it is very important to solve the contradiction that the coating agent with high-molecular weight causes the thickening of drilling fluid at a low temperature, while that with low-molecular weight causes a poor effect of encapsulation. Some international drilling companies have carried out a long-term research toward the wellbore stability of water sensitive shale and developed the amino group-based polymer hydration inhibitor that could enter the clay interlayer and inhibit the clay hydration dispersion (Akpan et al. 2019; Alsaba et al. 2020; Marin et al. 2009; Meng et al. 2019). However, the inhibitor does not have the coating effect, so it is unable to wrap the clay particles both on wellbore surface and in drilling cuttings to inhibit their hydration dispersion. Therefore, it needs to be used together with a coating agent. As currently reported, the molecular weight of the latest low-molecular-weight coating agent CAP was about 880,000 D. It was successfully applied in offshore areas, such as the South China Sea. Even though it improves the rheological properties of drilling fluids at low temperatures, the problem has not been completely solved. There are concerns about the compatibility of this cationic polymeric coating agent with other drilling fluid additives. Therefore, a coating agent which can meet the offshore, especially offshore deep-sea drilling requirement needs to be investigated.
The current paper aimed at simultaneous realization of strong encapsulation performance and excellent low-temperature rheological properties of the drilling fluid, as well as its good compatibility in drilling fluids. A kind of polymeric coating agent with low-molecular weight was developed for offshore deep-sea drilling fluids. Its structure and properties were analyzed in the study.
Materials and methods
Materials
Methacryloxyethyltrimethyl ammonium chloride and acrylamide were obtained from Wanduo Co., Ltd. 2-acrylamido-2-methylpropane sulfonic acid was obtained from Dingshengxin Chemicals. Ammonium persulfate, sodium hydrogen sulfite, and mercaptoacetic acid were obtained from Sinopharm Chemicals. All these materials were analytically pure. For the shale recovery rate experiment, the shale samples were obtained from offshore wells in the South China Sea.
Synthesis
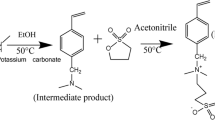
Acrylamide, methacryloxyethyltrimethyl ammonium chloride, and 2-acrylamido-2-methylpropane sulfonic acid at a mole ratio of 7:3:1 were added to a four-necked flask along with deionized water as the solvent. Oxygen was removed by purging the flask with nitrogen. The mixture was heated to 65 °C, and a mixture of sodium bisulfite and ammonium persulfate was added as the initiator under continuous nitrogen flow and at a constant temperature. Thioglycolic acid was added to terminate the reaction after 2.5 h. Then, the polymer product was purified with acetone and dried under vacuum.
Characterization
Fourier transformation infrared (FT-IR) analysis
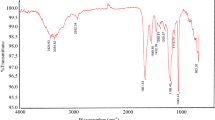
The FT-IR spectra of PADA were recorded by a NEXUS FT-IR spectrometer (Thermo Nicolet Corporation), scanning from 4000 to 400 cm−1 (Jin et al. 2019).
Determination of molecular weight
Gel Permeation Chromatography (Malvern 270 max GPC, Malvern Instruments, UK) was used to determine the molecular weight of PADA.
Performance evaluation of PADA
Shale recovery rate test
Shale recovery rate test is used to evaluate the dispersion of shale samples in water and drilling fluid. 280 mL test fluid and 40 g shale samples screened with a 10-mesh, were added into a roller oven cell. After hot rolling for 16 h, the solution containing shale samples were screened with a 40-mesh sieve. The remaining shale samples were collected, dried, and weighed, and the recovery rate was obtained (Xu et al. 2017).
Shale expansion experiment
The shale expansion experiment is used to analyse the hydration swelling property of shale samples in drilling fluids. The shale expansion experiments were conducted using a NP-2 expansion instrument to test the variations of the height of the shale sample to determine its linear expansion rate after immersed in pure water and coating agent solutions (Rana et al. 2020). Bentonite which was crushed and sifted through a 100-mesh screen, was used to prepare the core sample.
Transmission electron microscopy (TEM) analysis
TEM analysis was performed by JEM-2100UHR transmission electron microscope (JEOL, Japan) to study the structure of PADA-clay system.
Analysis of the particle size
The particle size distribution was tested by a Bettersize 2000 laser particle size analyzer (Dandong Bettersize instruments, China) to investigate the influence of PADA on clay particle size.
Analysis of zeta potential
The zeta potentials of clay suspensions containing 0.1 wt%, 0.3 wt%, 0.5 wt%, and 0.8 wt% PADA were measured using the zeta potential analyzer (Nano Brook Omni, Brook Heaven) in deionized water.
Interlayer spacing measurement
Mass of 2 g bentonite was added in 100 mL deionized water, and the dispersion was stirred for 24 h. Then PADA with different concentration was added into the dispersion, and the dispersion was stirred for another 24 h. After that, the mixture was centrifuged at 8000r/min for 20 min and washed to remove the un-adsorbed polymers. The precipitation was dried at 105 °C and grounded to fine powders. The interlayer space of the powders was measured by X-ray diffraction using an X’pert PRO MPD diffractometer (Zhong et al. 2016).
Rheological property tests
The rheological parameters of bentonite suspensions containing different coating agents were measured using a ZNN-D6 viscometer according to the API recommended standard procedure. The apparent viscosity (AV), plastic viscosity (PV), and yield point (YP) were obtained according to the following equations (Ismail et al. 2020).
Scanning electron microscope analysis of the filter cake
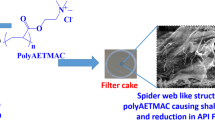
The filter cakes which were obtained by filtration tests with different drilling fluids were collected, and their surfaces were observed by S-4800 scanning electron microscope (Hitachi, Japan). The SEM images can indicate the effect of coating agent on the micro-structure of filter cake formed on the wellbore surface.
Biodegradability tests
The 5-day biochemical oxygen demand (BOD) and chemical oxygen demand (COD) were measured using Model 880 BOD testing instrument and HH-6 COD testing instrument (Jiangfen Electroanalytical Instrument Co., Ltd., Jiangsu, China), respectively. The BOD/COD ratio was used to analyse the biodegradability of the product (Zhang et al. 2017).
Characterization and performance evaluation of the coating agent
PADA characterization
Infrared spectrum analysis
The PADA infrared spectrum results are shown in Fig. 1. The peak at 3420 cm−1 was assigned to the –NH2 stretching vibration, and the peak at 1670 cm−1 was assigned to the characteristic –C=O adsorption, indicating that the final product contained acrylamide structural units. The –COO– absorption peak appeared at 1720 cm−1, and the –CH2– stretching vibration peak from the –CH2–N+(CH3)3 group appeared at 1451 cm−1, confirming that the methacryloxyethyltrimethyl ammonium chloride structural unit was also present in the polymer product. The peak at 1210 cm−1 was related to the –SO3– vibration absorption, indicating that the product contained 2-acrylamido-2-methylpropane sulfonic acid structural units.
Determination of molecular weight
The results of gel permeation chromatography show that the number-average molecular weight of PADA was 265,000 D. The value is much smaller than that of the CAP (about 880,000 D) and the traditional coating agent (over 1,500,000 D).
Shale inhibitive property and mechanism
Shale recovery rate experiment
The shale recovery rate experiment is the test method, which can reflect the wrapping up of the clay particles by coating agent and inhibitive effect on their dispersion. For the mass fractions of 0.3 wt% and 0.5 wt%, the shale recovery rates by aqueous solutions of PADA, current low-molecular-weight coating agent CAP, traditional high polymer coating agent HPAM, and PAM were tested, as shown in Table 1. The main components of shale sample #1 obtained from the offshore wells are quartz (47%), clay (24%), and calcite (11%), and the main components of sample #2 are quartz (48%), clay (22%), and anorthosite (10%). The results showed that the recovery rates were only 4.1% and 8.7% in water, respectively. These values indicated that the shale was highly prone to hydration dispersion and led to problems, such as wellbore instability and deterioration of drilling fluid performance. With the addition of coating agent, the shale recovery rate substantially increased. When the concentration was 0.3 wt%, the recovery rate of HPAM system was higher than that of other coating agents, while the recovery rate of PADA was similar to that of CAP, though better than that of the PAM. When the concentration was 0.5 wt%, the recovery rate of PADA was similar to that of HPAM and was slightly higher than that of CAP and significantly higher than that of the PAM. Therefore, with much lower molecular weight than that of other coating agents, PADA still had good inhibitive property for the hydration dispersion.
Shale expansion experiment
The coating agent could adsorb on the surface of clay minerals. As a result, the water molecules entering the clay slowed down. Due to this reason, the coating agent may also suppress the shale hydration expansion to some extent. The results of shale expansion experiments showed that the core expansion rate in pure water within 8 h was 42.1%, while the expansion rates were 22.7%, 21.2%, 18.1%, and 24.5% with 0.5 wt% PADA, 0.5 wt% CAP, 0.5 wt% HPAM, and 0.5 wt% PAM, respectively. Therefore, the coating agent has obvious inhibitive effect on clay hydration expansion. The effect of PADA is similar to those of CAP and PAM.
Transmission electron microscopy analysis
The micro-structure of PADA-clay system was observed using a transmission electron microscope. Figure 2a, b showed that, in the absence of PADA, the clay particles were well dispersed with particle size of about 1–2 μm. Figure 2c, d showed that after the addition of 0.3 wt% PADA, under the effects of polymer absorption and encapsulation, the size of clay particle increased, while the spacing obviously decreased, and the dispersion degree reduced. Multiple particles were accumulated, and the hydration dispersion was significantly reduced.
Particle size distribution and zeta potential analysis
Table 2 shows that after the addition of PADA, the size of clay particles significantly increased. When the concentration of PADA was 0.5 wt%, the particle size of clay increased by about 10 times. The particle size can be further increased by about 18 times when the concentration was 0.8 wt%. This indicated that the clay particles were coated and wrapped together by PADA.
The zeta potential test results (Fig. 3) showed that the effect of PADA in a low concentration on the zeta potential of bentonite system was small. This is due to the reason that PADA is a zwitterionic polymer. Under the combined interactions of cationic groups and sulfonic acid groups, the change of zeta potential of the system was not evident. When PADA concentration reached 0.8 wt%, the zeta potential reduced to − 23.07 mV. It was reported that the zeta potential from − 16 to − 30 mV was the threshold of weak dispersion condition for suspensions, and the zeta potential from − 10 to − 15 mV was the threshold of binding (Ritter and Geraut 1985). Therefore, although the electrostatic adsorption between PADA and clay occurred through the effect of cationic groups, the effect of electrical neutralization was not significant. The excessive electrical neutralization or even electrical reversal caused by the usage of cationic polymer coating agent will not occur, thus avoiding a decline in the stability of the drilling fluid system or a serious problem of flocculation caused by cationic polymers (Zhang et al. 2015).
Clay interlayer spacing analysis
Clay interlayer spacing was used to analyse the effects of the polymer additives on the hydration of clay minerals on micro scale. Figure 4 shows the variation in bentonite interlayer spacing with different added PADA concentrations. When the concentration of PADA was 0.1 wt%, the interlayer spacing increased from 1.33 to 1.39 nm, indicating that the polymer influenced the clay interlayer spacing. As the polymer concentration increased to 0.3 wt%, 0.5 wt%, and 0.8 wt%, the interlayer spacing was increased to 1.42 nm and then almost constant. Because the molecular weight of the PADA polymer was 265,000 D, the polymer was larger than the clay interlayer spacing and could not intercalate into the clay layers. The increase in interlayer spacing with the addition of PADA suggested that although the whole polymer molecule could not intercalate into the collection of clay particles, the cationic quaternary ammonium groups likely intercalated with the bentonite though electrostatic interactions. Therefore, the polymer adsorption could bond the adjacent lattices of clay particles together, thus inhibiting the hydration and dispersion of the clay particles.
Effect on the filter cake
Reducing the invasion of drilling fluid filtrates is important for stabilizing the wellbore, and a tight filter cake with low permeability is favored (Li et al. 2020). Generally, high-molecular-weight polymers, for example, HPAM at a low concentration, can help to form better mud cake on the wellbore surface because it can interact with bentonites and other additives (Adebayo and Bageri 2020). When the concentration is high, the quality of the filter cake will reduce due to the flocculation, and the filtrate loss will be increased. In order to investigate the effect of PADA on the structure of filter cake, high temperature and high pressure (130 °C/3.5 MPa) filtration tests were performed, and the SEM images of filter cake formed by different test fluids are shown in Fig. 5. It should be noted that the structure of the dried mud cake cannot fully reflect the downhole mud cake condition. Figure 5a, b show that the filter cake formed by 4 wt% bentonite suspension was not very tight, and there were some micro-pores in the surface. Therefore, the filter cake formed by bentonite particles alone is not tight enough to effectively retard the invasion of drilling fluid filtrates. For 4% bentonite suspension containing 0.3 wt% HPAM (Fig. 5c, d), the filter cake is tighter than that formed by bentonite alone, and there is no apparent micro-pores even in the SEM images with 3500-fold magnification. Therefore, the high-molecular polymer HPAM can help to increase the quality of the filter cake. In comparison, Fig. 5e, f show that the filter cake formed by PADA, which has much smaller molecular weight, was also tight without apparent micro-pores in the SEM images with 3500-fold magnification, indicating it has comparable effect with HAPM on improving the filter cake. Therefore, the addition of PADA at a low concentration into water-based drilling fluids can help to reduce the invasion of filtrates into the formation, thus decreasing the wellbore instability risk due to the hydration of clay minerals when encountering with the filtrates.
Analysis of coating mechanism
Based on the experiments above, the coating mechanism of PADA can be analyzed. The molecular chain of PADA contained both the cationic chain segment and the acrylamide chain segment with better flexibility. Through both the hydrogen bonding between the amide group and clay, and the electrostatic interaction between cationic group and clay, it absorbed onto the surface of clay particles and did not fall off easily. Part of the molecular chain, probably the quaternary ammonium group, intercalated onto the adjacent lattice of the clay particles by electrostatic interaction, thus bonding the lattice together to inhibit the hydration and dispersion of clay particles. The sulfonic acid group in molecular chain was ionized in water, so that the molecular chain carried a negative charge. Under the electrostatic repulsion and hydration film repulsion, the curled molecular chain was stretched (Kulkarni et al. 2005; Urata et al. 2004). It was advantageous to form multi-point adsorption on the surface of clay particles and coating them, so that the particles were bound together. Therefore, PADA had good coating and inhibition performances although its molecular chain is not such long as traditional coating agents like HPAM and PAM.
Evaluation of rheological properties at a low temperature
The key difference between offshore deep-sea drilling, and onshore or shallow water drilling is the existence of a low temperature interval of riser in deep-sea drilling at about 4 °C. The rheological properties of 4 wt% bentonite mud with additions of PADA, CAP, HPAM, and PAM in different concentrations were analyzed at 4 °C and 25 °C. The AV represented the total viscosity of the fluid and numerically equal to the sum of PV and YP. PV represented the viscosity which originated from the internal friction, while YP represented the viscosity resulting from the formations of spatial grid structures among clay particles and among high-molecular polymers in the fluid.
The results in Table 3 showed that with a concentration of 0.1 wt%, the AV of PADA system at 4 °C was the lowest among the 4 tested coating agent systems and had a value of only 13.5 mPa·s. For a concentration of 0.3 wt%, the viscosity of PADA system at 4 °C was still the lowest with its PV of 15 mPa·s, YP of 5.5 Pa, and AV of 20.5 mPa·s. Under same conditions, the HPAM system had PV of 38 mPa·s, YP of 17 Pa, and AV of 55 mPa·s, which were nearly 3 times of the values of the PADA system. The AVs of CAP system and PAM system were approximately 1.8 times and 1.7 times of that of PADA system, respectively. For the concentration of 0.5 wt%, the bentonite mud with additions of HPAM and PAM underwent apparent flocculation. Meanwhile, the AVs of PADA system and CAP system at 4 °C were 36 mPa·s and 51 mPa·s, respectively. Therefore, under same concentrations, the rheological parameters of bentonite mud with the addition of PADA were significantly lower than those of other coating agents. It was attributed to the low-molecular weight of PADA. The ratios of AV at 4 °C and 25 °C can be used to compare the degree of thickening of different coating agents. When the concentration of coating agents is 0.3 wt%, the ratios are 1.37, 1.44, 1.72, and 1.51 for PADA, CAP, HPAM, and PAM, respective, indicating that the thickening effect of PADA is weaker than other coating agents when the temperature was reduced from 25 to 4 °C, thus reducing the risks caused by the severe thickening of drilling fluid at a low temperature. Both the PV and YP of PADA system were significantly lower than those of other coating agent systems. Low PV indicated small internal friction among molecules at a low temperature for its short molecular chain. Low YP indicated that a large range of entanglement among PADA molecular chains did not occur to form the grid structure. This was beneficial to control the rheological properties of the drilling fluid and avoid the thickening of drilling fluid at a low temperature. Even if the concentration was higher than those of other coating agents, the PADA was still able to maintain a lower viscosity. The AV of 0.3 wt% PADA at 4 °C was 20.5 mPa·s, while the AVs of 0.1 wt% HPAM, 0.1 wt% CAP and 0.1 wt% PAM were 35 mPa·s, 26 mPa·s, and 20 mPa·s, respectively. The AV of 0.5 wt% PADA at 4 °C was 36 mPa·s, while the AVs of 0.3 wt% HPAM, 0.3 wt% CAP and 0.3 wt% PAM were at 55 mPa·s, 37.5 mPa·s, and 35.5 mPa·s, respectively. The results of Tables 1 and 3 also showed that the recovery rate of 0.5 wt% PADA solution was higher than that of 0.3 wt% HPAM solution, while the viscosity of 0.5 wt% PADA at a low temperature was obviously lower than that of 0.3 wt% HPAM. Therefore, the good shale inhibitive property and low-temperature rheological property of drilling fluids can be obtained simultaneously.
Biodegradability analysis
Aqueous solutions with 0.3 wt% and 0.5 wt% PADA were prepared for BOD (biochemical oxygen demand) and COD (chemical oxygen demand) tests, and the results are shown in Table 4. The BOD/COD ratios were 22.0% and 21.1% for 0.3 wt% PADA and 0.5 wt% PADA, respectively. According to the evaluation criterion for biodegradability, a BOD/COD ratio between 15 and 25% indicates that the sample is relatively easily biodegraded, and a ratio higher than 25% indicates the sample is easily biodegraded. Therefore, it can be concluded that PADA was relatively easy to biodegrade and considered an environmentally friendly coating agent (Yun et al. 2011).
Conclusions
A low-molecular-weight polymer coating agent named PADA was synthesized with the molecular weight of 265,000 D using acrylamide, methacryloxyethyltrimethyl ammonium chloride, and 2-acrylamido-2-methyl propane sulfonic acid as monomers. With much a lower molecular weight, PADA had similar effects to other coating agents including CAP and HPAM on the inhibition of the hydration dispersion of shales and performed better than PAM. PADA could coat and wrap the clay particles together, and thus significantly increased the clay particle size. The main inhibition mechanism was that the PADA could absorb onto the clay particles through both hydrogen bonding and electrostatic interactions and wrap them together. The cationic groups on the polymer chain intercalated onto the adjacent lattice of clay particles through electrostatic interactions, bonding the lattice together and inhibiting their hydration dispersion. At the same polymer concentration, the viscosity of bentonite mud with PADA was much lower than that of mud with other coating agents, especially at the low temperatures in deep sea. Therefore, the utilization of PADA could prevent the drilling fluid from seriously thickening at low temperatures as happens with traditional high-molecular-weight polymer coating agents. Moreover, PADA was relatively easily biodegraded.
References
Adebayo AR, Bageri BS (2020) A simple NMR methodology for evaluating filter cake properties and drilling fluid-induced formation damage. J Petrol Explor Prod Technol 10(4):1643–1655
Akpan EU, Enyi GC, Nasr G, Yahaya AA, Ahmadu AA, Saidu B (2019) Water-based drilling fluids for high-temperature applications and water-sensitive and dispersible shale formations. J Petrol Sci Eng 175:1028–1038
Alsaba M, Al Marshad A, Abbas A, Abdulkareem T, Al-Shammary A, Al-Ajmi M, Kebeish E (2020) Laboratory evaluation to assess the effectiveness of inhibitive nano-water-based drilling fluids for Zubair shale formation. J Petrol Explor Prod Technol 10(2):419–428
Davison J, Clary S, Saasen A, Allouche M, Bodin D, and Nguyen V (1999) Rheology of various drilling fluid systems under deepwater drilling conditions and the importance of accurate predictions of downhole fluid hydraulics. In: SPE annual technical conference and exhibition, 1999. Society of Petroleum Engineers
Dokhani V, Ma Y, Yu M (2016) Determination of equivalent circulating density of drilling fluids in deepwater drilling. J Nat Gas Sci Eng 34:1096–1105
Herzhaft B, Peysson Y, Isambourg P, Delepoulle A, and Abdoulaye T (2001) Rheological properties of drilling muds in deep offshore conditions. In SPE/IADC drilling conference, 2001. Society of Petroleum Engineers
Ismail AR, Mohd NM, Basir NF, Oseh JO, Ismail I, Blkoor SO (2020) Improvement of rheological and filtration characteristics of water-based drilling fluids using naturally derived henna leaf and hibiscus leaf extracts. J Petrol Explor Prod Technol 10(8):3541–3556
Jain R, Mahto V (2015) Evaluation of polyacrylamide/clay composite as a potential drilling fluid additive in inhibitive water based drilling fluid system. J Petrol Sci Eng 133:612–621
Jin J, Wang H, Jing Y, Liu M, Wang D, Li Y, Bao M (2019) An efficient and environmental-friendly dispersant based on the synergy of amphiphilic surfactants for oil spill remediation. Chemosphere 215:241–247
Kadaster A, Guild G, Hanni G, Schmidt D (1992) Field applications of PHPA muds. SPE Drill Eng 7(03):191–199
Knox D, Bulgachev R, and Cameron I (2015) Defining fragile-the challenge of engineering drilling fluids for narrow ECD windows. In: SPE/IADC drilling conference and exhibition, 2015. Society of Petroleum Engineers
Kulkarni MV, Viswanath AK, Aiyer R, Khanna P (2005) Synthesis, characterization, and morphology of p-toluene sulfonic acid-doped polyaniline: a material for humidity sensing application. J Polym Sci Part b: Polym Phys 43(16):2161–2169
Li P, Xu Y, Liu Y, Feng J, Hui B, Feng Y, Hu M, Guo J (2020) Terpolymer with rigid side chain as filtrate reducer for water-based drilling fluids. J Appl Polym Sci 138(16):50237
Liu K, Ostadhassan M, Xu X (2020) A comparison study of the unloading behavior in shale samples in nanoindentation experiments using different models. J Petrol Sci Eng 186:106715
Marin JU, Shah F, Serrano MA, Jaramillo A, Arevalo W, and Priandi GB (2009) First deepwater well successfully drilled in Colombia with a high-performance water-based fluid. In: Latin American and Caribbean petroleum engineering conference, 2009. Society of Petroleum Engineers
Mehtar MA, Mielke SK, Alfonzo NE, Young S, Brangetto M, and Soliman AA (2010) Effective implementation of high performance water based fluid provides superior shale stability offshore Abu Dhabi. In: Abu Dhabi international petroleum exhibition and conference, 2010. Society of Petroleum Engineers
Meng M, Chen P, Ren R (2019) Statistic evaluation of failure criteria in wellbore stability with temperature effects. Fuel 252:730–752
Rana A, Khan I, Ali S, Saleh TA, Khan SA (2020) Controlling shale swelling and fluid loss properties of water-based drilling mud via ultrasonic impregnated SWCNTs/PVP nanocomposites. Energy Fuels 34(8):9515–9523
Ritter A, and Geraut R (1985) New optimization drilling fluid programs for reactive shale formations. In: SPE annual technical conference and exhibition, 1985. Society of Petroleum Engineers
Swai RE (2020) A review of molecular dynamics simulations in the designing of effective shale inhibitors: application for drilling with water-based drilling fluids. J Petrol Explor Prod Technol 10:3515–3532
Urata S, Irisawa J, Takada A, Tsuzuki S, Shinoda W, Mikami M (2004) Intermolecular interaction between the pendant chain of perfluorinated ionomer and water. Phys Chem Chem Phys 6(13):3325–3332
Van Oort E (2003) On the physical and chemical stability of shales. J Petrol Sci Eng 38(3):213–235
Xu J, Qiu Z, Huang W, Zhao X (2017) Preparation and performance properties of polymer latex SDNL in water-based drilling fluids for drilling troublesome shale formations. J Nat Gas Sci Eng 37:462–470
Yun M, Fangling Q, Chunyan T (2011) Application of flocculation-fenton oxidation-sbr process on treating oil field fracturing wastewater. Chem Eng Oil Gas 1:026
Zamora M, Broussard PN, and Stephens MP (2000) The top 10 mud-related concerns in deepwater drilling operations, 2000
Zhang H, Xiong Z, Ji F, Lai B, Yang P (2017) Pretreatment of shale gas drilling flowback fluid (SGDF) by the microscale Fe0/persulfate/O3 process (mFe0/PS/O3). Chemosphere 176:192–201
Zhang Y, Miao Z, Zou J (2015) A new cation-modified Al-polyacrylamide flocculant for solid–liquid separation in waste drilling fluid. J Appl Polym Sci 132(11):41641
Zhao X, Qiu Z, Gao J, Ren X, Li J, Huang W (2021) Mechanism and effect of nanoparticles on controlling fines migration in unconsolidated sandstone formations. SPE J: SPE-204474-PA. https://doi.org/10.2118/204474-PA
Zhao X, Qiu Z, Zhang Y, Zhong H, Huang W, Tang Z (2017) Zwitterionic polymer P (AM-DMC-AMPS) as a low-molecular-weight encapsulator in deepwater drilling fluid. Appl Sci 7(6):594
Zhong H, Qiu Z, Tang Z, Zhang X, Xu J, Huang W (2016) Study of 4, 4′-methylenebis-cyclohexanamine as a high temperature-resistant shale inhibitor. J Mater Sci 51(16):7585–7597
Funding
None.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ren, X., Liu, R. & Ma, Z. Experimental investigation of a low-molecular-weight polymer coating agent for deep-sea oil and gas drilling. J Petrol Explor Prod Technol 11, 2953–2962 (2021). https://doi.org/10.1007/s13202-021-01198-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13202-021-01198-y