Abstract
Water injection technique is contributing millions of oil barrels on a daily basis to the global oil supply; one of the challenges faced by such an important production method is scale buildup due to the different dissolved solids and high salinity of the injected water. For produced water or effluent water, pretreatment is a critical step before the injection process to reduce the risk of scale potential and well formation damage. One of the beneficial treatments of the effluent water is to be mixed in a compatible ratio with seawater, especially for those countries with easy access to seawater. Thus, the objective is to find a compatible mix ratio with seawater as one way of solving effluent water reuse without increasing the risk of scale and formation damage during water injection operation. In this study, effluent water samples were collected from different oilfield geographical locations in Kuwait, and then the samples were analyzed in the laboratory for their physical and chemical properties to select the most compatible one for mixing with seawater. The selected effluent water was then mixed with seawater in 1:1 ratio to evaluate the compatibility of the mix in terms of sulfate formation and scale tendency. The selected North field/seawater was tested under 75 ºC and 1000 PSi in an autoclave. The filtered precipitates were characterized by energy-dispersive spectroscopy (EDS) and scanning electronic microscope (SEM) micrographs. Further, the formations of sulfate were detected by X-ray diffraction (XRD). The OLI ScaleChem software was used to investigate the composition of mineral scales that may occur in the water mix at different ratios ranging from 0 to 100% to compare it with the actual water mix finding. In the effluent waters comparison part, North field water showed low pH (4.6) and alkalinity, as well as moderate TDS (190,000 MG/L). The main constituents of the scale deposited at the selected water mixing ratio were calcium sulfate CaSO4 and silicon oxide detected by XRD. However, the amount of barium sulfate was not presented in the precipitates indicating that all of it went in the liquid phase. The study concludes that at downhole conditions, the compatible mix of North Kuwait effluent water with seawater has high risk of barium sulfate in comparison with other common scale such as calcium sulfate, calcium carbonate and strontium sulfate (SrSO4). Also the study revealed that the prediction of different seawater ratios showed that at 70% seawater concentration, the barium sulfate concentration drops and consequently the scale risk reduced.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Oil field problems are continuously occurring due to the complexity of this industry and the related environmental issues. Some of these problems are solvable, others are controllable, and many remain a challenge. Most of Kuwait’s major producing fields are over 60 years old; therefore, field maturity and production techniques are becoming a concern. As a result of maturing oilfields, KOC have to deal with the same two-headed problem the whole world is facing on how to handle the increased amount of produced water. Water, oil and gas usually coexist in the petroleum reservoirs, and therefore when oil is produced associated water produced in different percentages depends on the technology, age of the well and formation. Kuwait has different oilfields distributed according to the geographical location. The North field, West field, South and East Field are approximately at 40% water cut. The new target production in 2030 of a four million bbl/day increase in produced water can reach to a level of 1.5 million bbl/day (Alfarhan and Duane 2011; Nabzar 2011). As regulations have become more stringent, the disposal method costs increase, and produced water becomes abundant and beneficial reuse is becoming a more viable option.
Many studies have shown that produced water has high TDS content, which is major cause of scale formation and pipe clogging (Merdhah and Yassin 2007a, 2007b, 2007c; Arthur and Bruce 2005; Al-Ghouti et al. 2019). It is well documented that oilfield scales are inorganic deposits formed due to the precipitation of solids resulting from brines that are present in the reservoir and production system. Scale buildup is a major factor that controls the selection of water type that can be used for Enhancing Oil recovery application (EOR), namely water injection (Merdhah and Yassi 2007a, 2007b, 2007c; Hadia et al. 2008; Kokal and Al-Kaabi 2010). When two incompatible waters are mixed, it causes changes in the super-saturation of the mixture forms which leads to precipitation of scales (Ghalib and Almallah 2017). The individual waters may be quite stable under all system conditions and present no scale problems. Ali Roostai 2016 reported that once different waters are mixed, the reaction between ions dissolved in the individual waters may form insoluble products that cause permeability damage in the vicinity of the wellbore. Thus, selection of the type of water for water injection is a critical task and has to be studied before starting the injection. Further, suspended solids in the water that are known for their contribution to erosion can be considered a source for scale risk as these solids such as silicon oxide help in the scale buildup in the system. For environmental reasons, many oil companies try to mix produced water with other water forming a compatible mix for injection purposes. However, the main concern in the mix selection is to reduce the scale tendency and avoid well formation damage. The most common scales formed in the oil and gas industries are calcium carbonate (CaCO3), barium sulfate (BaSO4) and strontium sulfate (El Hajj et al. 2015). Others may exist but have lower impact such as ferrous carbonate and ferrous sulfide. All deposits could be formed during secondary recovery when high-salinity water is used to increase the pressure in the reservoir, knowing that formation water contains cations (Ba2+, Ca2+, Sr2+) and seawater contains SO42-ions.
Although BaSO4 is rarely formed when compared with other sulfate scales, it is particularly tenacious and resistant to acid treatment. Also, BaSO4 is considered to be the most troublesome salt deposit because of its low solubility limits and the deposits are hard and compact (Bader 2006; Merdhah and Yassin 2007a, 2007b, 2007c; Merdhah 2012). These inorganic deposits have been seen over the years as the major cause of formation damage either in injection or in production (Adhia and Bachtiar 2011; SPE 2015; Civan 2015). In addition, it influences equipment wear and corrosion, restricts flow and causes a reduction in heat exchanger efficiency. This leads to an increase in maintenance costs, emergency shutdown, reduction in production and production–equipment failures; thus increasing the operational and production costs. Scales are usually formed in perforation and tubes, mostly where the temperature and/or pressure is very low (Davidzon 2007, 2008). Due to the critical impact of scale on well production and maintenance, many softwares were developed to predict scale mass and scale tendency for fluids used in the oil fields (Kan et al. 2019). These software are basically developed by semi-empirically modeling the thermodynamic parameters using data from liquids analysis and chemical properties in coordination with temperature, pressure and ionic strength (Kan and Tomson 2012). These softwares are expected to play a major role in scale prevention to ensure continuous production from existing reserves that produce brine or use brine for water treatment.
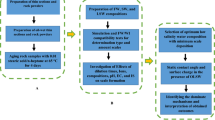
This work sheds light on a novel and systematic way of dealing with the increasingly produced water from the oil field in countries with abundant seawater. The paper has been undertaken to experimentally identify the composition of mineral scales that occur in effluent water collected from different oil fields in Kuwait. It systematically investigates water compatibility by mixing seawater with effluent water collected from NK oilfield in 1:1 ratio and reports their performance in terms of scale formation. In addition, the scale tendency also was investigated using the OLI ScaleChem software to identify the composition of mineral scales that may occur in the North field water mix during injection and predict the best ratio of seawater/effluent water mix that leads to minimal formation damage, scale risk reduction, cost of handling and environmental consequences.
Materials and experimental procedure
Site and materials
Effluent water was sampled from various oil fields in Kuwait (from the North (NK), South (SK), West (WK) and Southwest (SWK) oilfields). Three glass bottles of 500 ml volume were used for the sampling purpose from each field. Both seawater and effluent water samples were analyzed in the Petroleum Research Center Laboratories, KISR. The physical characteristics of the water sampled were determined by Inductive Couple Plasma (ICP) analysis to determine the composition of dissolved solids, such as magnesium, sodium, potassium, iron, strontium, barium, lithium and boron. The samples were subjected to approximate dilution for effective analysis, and 4-point calibration was carried out as per ASTM D-1976-12 with all standards for analysis.
Procedure; water selection and preparation
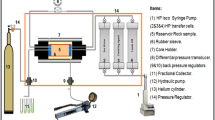
The detailed physical characterization of water from these oilfields is necessary in predicting the possible impact of water on scaling and corrosivity impact. After water sampling from different fields and characterization, in the second part of the experimental procedure, the selected effluent water (NK) based on analysis results was mixed with seawater (1:1) in an autoclave cell with 1.2 l total amount. The system was purged with nitrogen to remove air bubbles before setting the pressure at 1000 psi and a temperature at 75 °C and the controller and the stirrer to 60 RPM and left for 3 h until temperature stabilization. After 24 h, the stirrer was switched off, temperature was decreased to 25 °C and the pressure was released carefully (Funderburg 2016; Satterfield 2005).
Parameters and data collection
Later in the testing procedure, the water mix was subjected to downhole conditions (75 ºC and 1000 psi); samples were collected, and the filtered precipitates were characterized by energy-dispersive spectroscopy (EDS) and X-ray diffraction (XRD) to get the information about crystal structure, where EDS gives the information about composition analyses. Scanning electron microscope (SEM) was used for obtaining micrographs of the precipitates. OLI Studio version 9.6 is used to calculate the chemical and phase behavior of the water mixture of inorganic and organic chemicals, for predicting scale mass and scale tendency.
Statistical analysis
The experiment was conducted based on three samples per field, and the results were analyzed statistically with the use of an average of three techniques with less than 1% error in results.
Results and discussion
Effluent water comparison and selection
The high salt concentrations in brine come from salt deposits in oil-producing formations, where overall salinity and concentrations of sulfates can vary widely by location nature of formation. Kuwaiti effluent water has very high total dissolved solids (TDS) and salinity in comparison with other oil fields such as Qatar, Iraq, and USA (AlAnezi et al. 2018); thus, it is classified as brine. Four Kuwaiti oil fields, namely South Kuwait, North Kuwait, East Kuwait and West Kuwait, were investigated for their effluent water properties. The different oilfields have shown different properties in terms of sulfate, pH and other properties, as illustrated in Table 1. Analysis showed that effluent water from North Kuwait had the lowest pH and conductivity, while those from South Kuwait had the lowest readings for the rest of the physical characteristics. The produced water samples collected from West Kuwait had the highest readings for all physical characteristics, except alkalinity. Figure 1 shows the difference in alkalinity and TDS; it can be noticed that NK has a very low alkalinity, less than 16 MG/L in comparison with SK that has over 350 MG/L alkalinity. This low value of alkalinity is considered very harmful on pipes (Lytle and Schock 2008) as well as the corrosive potential risk increases with the expected increase in water pH. This parameter is critical when it is related to mixing with seawater as the level of alkalinity should be in the range of less than 80 MG/L after mixing to reduce corrosion risk in pipes. Moreover, level of TDS for all selected fields is at a close range 150,000–220,000 MG/L. Nonetheless the NK TDS value is considered the lowest except for SK (which has a very high alkalinity).
Therefore, the main objective of this novel part of the study is to select the most suitable effluent water from the tested fields to mix with seawater then verify it by the OLI software. The comparison of effluent water properties for the nominated oilfields resulted in selecting NK effluent water to be mixed with seawater for water injection purpose based on pH, TDS and alkalinity results (Tam and Elefsiniotis 2009).
Compatible water properties
-
EDS, SEM and XRD analysis
Various parameters such as ionic composition of the water, chemical incompatibilities as in scales, precipitates and suspended solid content were described as the potential quality factors in the injection water process (Igunnu and Chen 2014). The obtained results from this part of the study indicating that the main constituents of the scale deposited at the selected water mixing ratio were strontium sulfate, calcium carbonate and ferrous carbonate. Figure 2 represents EDS analysis for seawater/NK effluent water mix (1:1), the position of a peak in the spectrum, its energy, and identifies the element; the area under the peak is proportional to the number of atoms of the element in the irradiated area. Fe peak is clearly seen, but it is recognized as common impurity in such water samples. Therefore, it is not considered as a major parameter to assist the compatibility of the prepared mix although it may form some ferrous oxide. EDS also detected Si and Ni traces which may be attributed to the calcifying medium containing these ions. The low intensity of Ca in other peaks in the graph can be attributed to a small amount of calcium being present in the compound or different bonds.
Moreover, EDS peaks show that barium has not precipitated and all of it went in the liquid phase (water) which indicate that the concentration of barium will increase in the injection water in comparison with other elements such as calcium which was filtered in the solid form. Consequently, this can increase the chance of barium sulfate formation over other sulfates, where such increase synergized with the predicted results for scale tendency using OLI software as it will be discussed later in 3.3 section. However, the high levels of barium sulfate can be attributed to elevated level of strontium calcium iron and sodium positive ions or chloride and bromide anions.
Scales and precipitates can be formed in waters from a number of root causes. Changes in temperature and pressure of the water, as it mixes with other water of a different nature, may initiate pH changes, hence may begin the formation of scales (Li et al. 2013) as pH change is an indication of chemical changes in the media. The filtered precipitates from the prepared samples were examined by SEM to observe the particle size and morphology of the precipitates after being subjected to elevated temperature 75 ºC and pressure 1000 Psi. This is a very critical step in the investigation to give a better understanding of the chemical behavior for the selected water under the operating conditions. The formations of CaSO4 and SrSO4 were detected by SEM micrographs shown in Fig. 3, which indicates a possible formation of scale. This finding synergized with the results, produced from XRD, especially the presence of calcium sulfate.
In Fig. 4, The XRD analysis showed that the calcium was present in the form of calcium sulfate in addition to some traces of silicon oxide. This finding confirms that barium sulfate went with liquid and did not precipitate or accumulate during the mixing process even at downhole conditions. Such findings illustrate that the prepared mix is compatible in a way of not accumulating barium sulfate which is the number one cause for scale in oilfields; however, as it went with the liquid it may part with the underground water minerals and form insoluble mix.
Scale tendency prediction
The objective of this part of the study is to compare the experimental finding with the prediction results from OLI software.
A range of waters mix ratio (seawater/effluent) was selected for scale tendency evaluation using the OLI software. Figure 5 shows that at 55% concentration of seawater, the mix starts to reduce the barium sulfate in the compatible water mix; this result synergizes with the results obtained from XRD and EDS where both showed scale mass at the selected water mix percentage at acceptable levels (lower than effluent water), while the highest combined inorganic scale precipitation was observed at approximately 20% ratio for the effluent–seawater mixture. When the prediction results were compared with the experimental finding, it can be noticed that at 1:1 ratio the mix is considered compatible but has high potential of sulfates risk depending on the nature of the formation salinity of the underground water. Thus, a higher percentage up to 70% of seawater in the mix is preferable and considered more compatible (in terms of scale risk) for water injection purpose. Also, it is likely that as the water is exposed to more sulfates in the environment (well water) the barium would precipitate out as barium sulfate; other risks associated with barium are forming insoluble mix. In addition to lowering scale tendency by adjusting alkalinity, seawater is also known for given better oil recovery than brine water which makes the selection of 70–75% of seawater in the water mix favorable for both scale reduction and oil recovery (Austad et al. 2010). Moreover, Table 2 shows that calcium carbonate and strontium sulfate are of a less scale tendency value when compared to the barium sulfate which is reflected in Table 3.
Conclusion
The water compatibility experiments for the reuse of produced water have shown that Kuwaiti Northern field has suitable effluent water for mixing with seawater better than other effluent water from the tested locations. The alkalinity level and TDS remained in acceptable levels after mixing with seawater in 1:1 ratio. Scales and precipitates can be formed in waters from a number of root causes. Changes in temperature and pressure of the waters, as it mixes with other water of a different nature, may initiate pH changes, hence may begin the formation of scales or contribute to scale buildup. EDS and XRD results have shown that the barium sulfate which is considered a top cause for scale in the oil field was at a very small concentration level in the solid precipitates after mixing in an environment simulating downhole conditions, suggesting that barium sulfate went with liquid and did not precipitate or accumulate during the mixing process even at the downhole conditions which expand the risk of forming insoluble mix with underground minerals during the injection process. Using the OLI prediction was a critical step in this study to compare the experimental results with the scale tendency and scale mass results from the software; the prediction indicated that calcium carbonate and strontium sulfate are of a less scale tendency values when compared to the barium sulfate which is reflected in the same pattern before and after subjecting to downhole conditions. The prediction confirmed that seawater is less harsh on scale than NK effluent water as brine has a high salt concentration recorded up to 10 times the salinity of ocean water. In general, this work reveals that scale risk can be minimized by mixing NK effluent with seawater at optimum ratio. This finding is very critical in terms of maintenance cost and produced water treatment since North fields in Kuwait are currently applying water injection to most of their wells.
References
Al-Ghouti MA, Al-Kaabi M, Ashfaq M (2019) Produced water characteristics, treatment and reuse: a review. J Water Process Eng 28:222–239
AlAnezi K, Al-Samhan M, Belkharchouche M (2018) Comparative analysis of produced water collected from different oil gathering centers in Kuwait. J Environ Protect 9(6):ID 85066
Alfarhan A, Duane M (2011) Geochemistry and modification of oilfield brines in surface pits in northern Kuwait. Arab J Geosci 5:1055–1068
Arthur J, Bruce G (2005) Technical summary of oil&gas produced watertreatment technologies. All Consulting, LLC, Tulsa
Austad T, Rezaeidoust A, Puntervold T (2010) Chemical mechanism of low salinity water flooding in sandstone reservoirs. Presented at the SPE Improved Oil Recovery Symposium, Tulsa, Oklahoma, USA
Bader M (2006) Sulfate scale problems in oil fields water injection operations. Desalination 201(1):100–105
Civan F (2015) Reservoir Formation Damage, Book
Davidzon M (2007) The scale formation in tubes under a constant heat load. Teploenergetika 5:64–67
Davidzon M (2008) Scale formation in waterwall tubes of boilers. Thermal Eng 55(7):582–586
El Hajj H, Pal O, Zoghbi B (2015) Compositional analysis and treatment of oilfield scales. In: SPE Saudi Arabia Section Annual Technical Symposium, SPE-178002-MS
Funderburg E (2016) Jar test helps determine compatible chemical mixes, Report. Nobel Research Institute, Norway
Ghalib H, Almallah I (2017) Scaling simulation resulting from mixing predicted model between Mishrif formation water and different waters injection in Basrah oil field, southern Iraq”. Model Earth Syst Environ 3:1557
Hadia N, Chaudhari L, Mitra SK, Vinjamur M, Singh R (2008) Effect of scaling parameters on waterflood performance with horizontal and vertical wells. Energy Fuels 22:402–409
Igunnu ET, Chen GZ (2014) Produced water treatment technologies. Int J Low-Carbon Technol 9:157–177
Adhia M, Bachtiar A (2011) Preliminarystudy and identificationof injection water compatibility for west Seno pressure maintenance project. In: Proceeding, indonesia petroleum association
Kan A, Tomson M (2012) Scale prediction for oil and gas production. SPE J 17(2):362–378
Kan A, Dai J, Dang G (2019) Recent advances in scale prediction: approach and limitations. SPE J, print.
Kokal S, Al-Kaabi A (2010) Enhanced oil recovery: challenges and opportunities. EXPEC Advanced Research Center, Saudi Aramco, Dhahran
Li H, Liu L, Li M, Zhang X (2013) Effects of pH, temperature, dissolved oxygen, and flow rate on phosphorus release processes at the sediment and water interface in storm sewer. J Anal Methods Chem Article ID 104316
Lytle DA, Schock MR (2008) Pitting corrosion of copper in waters with high ph and low alkalinity. J Am Water Works Assoc 100(3):115–128
Merdhah A (2012) Inhibition of barium sulfate scale at high-barium formation water. J Pet Sci Eng 90:124–130
Merdhah A, Yassin A (2007a) scale formation in oil reservoir during water injection at high-salinity formation water. J Appl Sci 7(21):3198–3207
Merdhah A, Yassin A (2007b) Study of Scale Formation in Oil Reservoir During Water injection-A review. In: Marine science &technology seminar, pp 22–23
Merdhah A, Yassin A (2007c) barium sulfate scale formation in oil reservoir during water injection at high-barium formation water. J Appl Sci 7(17):2393–2403
Nabzar L (2011) Panorama: water in fuel production oil production and refining. INIS 42:20
Roostai A (2016) An experimental investigation of different formation waters and injection water incompatibility to obtain the optimum water mixing ratio in injection processe. J Pet Sci Technol 6(1):63–72
SPE, Formation damage (2015) Technical Report, Category:YR
Satterfield Z (2005) Jar Testing, Publishedby the NATIONAL ENVIRONMENTAL SERVICES CENTER, vol 5, no 1, p 105
Tam YS, Elefsiniotis P (2009) Corrosion control in water supply systems: effect of pH, alkalinity, and orthophosphate on lead and copper leaching from brass plumbing. J Environ Sci Health Part A 44(12):1251–1260
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Al-Samhan, M., Alanezi, K., Al-Fadhli, J. et al. Evaluating scale deposition and scale tendency of effluent water mix with seawater for compatible injection water. J Petrol Explor Prod Technol 10, 2105–2111 (2020). https://doi.org/10.1007/s13202-020-00849-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13202-020-00849-w