Abstract
This study explores seasonal fluctuations in water quality and phytoplankton communities within Karun-4 Dam Lake, the largest double-arch dam in the Middle East situated in Iran. Employing a systematic approach, 26 sampling stations were strategically selected to collect surface water samples at a depth of 50 cm during the midpoints of each season throughout 2019. Significant seasonal variations in water quality parameters and phytoplankton composition were observed. Predominant species included Chrysophyceae (38%) and Bacillariophyceae (32%), indicative of nutrient-rich conditions, particularly during spring and summer, as evidenced by the eutrophic state (Carlson Trophic State Index: 59.43 and 53.96, respectively). Summer exhibited the highest diversity (Shannon–Wiener Index = 2.27) and lowest evenness (Pielou’s Evenness Index = 0.21). PCA and CCA analyses revealed season-specific preferences for nutrients and ions among phytoplankton species. Water temperature emerged as a crucial factor in spring and summer, while environments with elevated bicarbonate and alkalinity levels were less favorable during winter and fall. This study provides essential insights into Karun-4 Lake's dynamic ecological conditions, underscoring the necessity for ongoing monitoring to discern long-term trends and anthropogenic impacts for effective ecosystem management.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Acting as the backbone of human civilization, freshwater resources have sustained agricultural practices throughout history and currently fuel diverse human developments worldwide. Encompassing both groundwater and surface water, they only constitute only nearly one percent of the total water available on Earth, distributed unevenly across the globe and rapidly diminishing due to changing global climate in many regions such as Iran and Iraq in the Middle East (Moshir Panahi et al. 2020; Salman et al. 2021) and Egypt in North Africa (Abd Ellah 2020). It is estimated that per capita freshwater availability has been halved since the mid-twentieth century (Buta et al. 2023). Furthermore, large-scale remote sensing analysis revealed a decline in the quality of inland water worldwide, affecting over 60% of the 2000 major global inland waters (Wang et al. 2018). The concurrent decline in both quantity and quality emphasizes the urgent need for strict management and conservation of freshwater resources to meet the growing demands of human activities, especially in arid countries witnessing high human pressures (Kazemi et al. 2019).
To take full advantage of freshwater resources, humans have constructed artificial reservoirs to store surface freshwater, providing reasonable allocations and further expansion of developments all across parts of the world, especially in arid regions (Moshir Panahi et al. 2020). First, these constructions can bring about hydromorphological and geomorphological alterations to basins. Moreover, while the water quality in these reservoirs may be inconsequential for certain applications such as flood protection and hydropower, it becomes salient when humans are direct or indirect users of the water (Wassie and Melese 2017). Consequently, freshwater in these constructions should remain unpolluted or reach required standards for human use, safeguarding against disturbances arising from natural or anthropogenic alterations in chemical characteristics, such as pH, phosphorus, nitrite, nitrate, ammonia, and dissolved oxygen (Singh et al. 2019; Patale and Tank 2022). Due to the influx of organic compounds enriched with phosphorus and nitrogen, reservoirs also experience increased fertility, also known as eutrophication (Saturday et al. 2023), allowing undesirable species such as cyanobacteria, diatoms, and green algae to dominate the water and lead to water bloom (Li et al. 2022). This process impairs the functioning and resilience of aquatic ecosystems and the suitability of water for human activities (Buta et al. 2023), occurring not only as a result of oxygen consumption for photosynthesis but also the release of toxic metabolic products by some species (Glibert 2017).
The degradation of water quality hinges on the most important controlling factor, its temporal variation and relation to other factors such as climate parameters, hydrological events, and the intensity of human activities such as agriculture (Shahradnia et al. 2021, 2022). This underscores the significance of spatio-temporal analyses and the continuous monitoring of water quality parameters. Illustratively, Ni et al. (2018) explored the connections between water quality and the phytoplankton community in an intertidal mudflat pond in Hangzhou Bay. Their findings revealed significant correlations between most phytoplankton species and environmental factors such as temperature, pH, and dissolved oxygen. Examining the spatio-temporal evolution of eutrophication and water quality in the Turawa dam reservoir, Poland, Buta et al. (2023) confirmed the ongoing eutrophication, classifying the reservoir as either eutrophic or mesotrophic based on trophic status indices. Asadian et al. (2020) also classified the Zayandeh River dam lake as oligotrophic during spring and summer, possibly attributed to floods, runoff, and farmland drainage in the region. Investigating phytoplankton communities in 30 rubber dams over a 2-year field monitoring, Bao et al. (2022) observed dominance of Bacillariophyta in natural areas throughout the year, with Cyanophyta and Chlorophyta showing significant increases during warm seasons. Gecheva et al. (2020) found elevated nutrient levels in three standing water bodies in Bulgaria during summer, categorizing them as either eu- or hypertrophic. Moreover, they reported the highest phytoplankton species count and functional diversity in the meso-eutrophic condition.
The Karun-4 Dam Lake stands out as a pivotal water reservoir in western Iran, playing a crucial role in the regulation and distribution of a substantial share of surface freshwater within the Zagros mountain chain. Due to the extensive utilization of the lake water for various human activities, it becomes imperative to meticulously monitor and conserve its water quality. To address this objective, the present study conducted an assessment encompassing a range of water quality parameters throughout the four seasons of the year 2019. In addition to scrutinizing the chemical attributes of the water, efforts were made to identify the key chemical characteristics governing the water quality in the region. Furthermore, an analysis of phytoplankton communities was undertaken for each season to gauge their diversity and dominance. Ultimately, the trophic status of the lake was calculated for each season, and the factors influencing the presence of each species were measured and presented.
Material and methods
Experiment sites
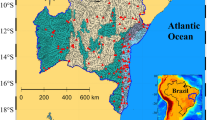
The Karun-4 Dam Lake (hereafter Karun Lake) is located in Chaharmahal-va-Bakhtiari Province, western Iran. It spans the main branches of the Karun River and currently stands as the largest double-arch dam in the Middle East (Fig. 1). The construction of the Karun Dam (31° 36′ N, 50° 24′ E) concluded in March 2010, resulting in a water level of 1025 m and a capacity of 2.2 billion m3 (Ashjari et al. 2019). The primary objectives of the dam include the provision of agricultural water for downstream lands, the generation of hydropower electricity (2100 gigawatt hours), and the regulation of potentially devastating seasonal floods. The average annual precipitation in the reservoir's surrounding areas is estimated to be approximately 680 mm. The minimum and maximum air temperatures at the site are recorded as 8.0 and 32.4 °C, respectively (Statistical Center of Iran 2016).
Sample collection
In this investigation, 26 sampling stations were selected systematically and evenly distributed across the entire expanse of the lake's surface water (Fig. 1) for the assessment of water phytoplankton communities and chemical parameters. Sample collection occurred on four occasions throughout the year 2019, precisely on the midpoint of each season. All samples were acquired between 9:00 and 11:00 a.m. For this purpose, around 1 L of water was withdrawn from a depth of 50 cm, utilizing polyethylene containers that were pre-rinsed with 10% nitric acid, and care was taken to avoid any contact with the hands of the sampling personnel.
Measurement of water chemical characteristics and chlorophyll-a
Water temperature (T), dissolved oxygen (O2), electrical conductivity (EC), and pH were measured on site using the portable HQ40D Digital multi-meter kit. The measurement of lake trophic conditions necessitated the assessment of water transparency, accomplished through the utilization of the Secchi disk depth device. Turbidity (TUR) was also determined with a turbidity meter (2100N HACH Turbidity meter). Alkalinity (ALK) and the ions calcium (Ca2+) and chloride (Cl−) were determined through the titration method (Rice et al. 2012). Sodium (Na+) and potassium (K+) were measured using a photometer. Fluoride (F−), magnesium (Mg2+), ammonium (\({\text{NH}}_{4}^{+}\)), iron (Fe2+), potassium (K+), sulfate (\({\text{SO}}_{4}^{2-}\)), nitrate (\({\text{NO}}_{3}^{-}\)), and phosphate (\({\text{PO}}_{4}^{3-}\)) together with the compound silicon dioxide (SiO2) were quantified using the powder pillow procedures with a spectrophotometer (DR5000 HACH).
To quantify the concentration of chlorophyll-a (Chl-a), the water sample underwent initial agitation. Subsequently, a defined volume of water, determined by its color, was subjected to filtration using Whatman filter papers. Following this, Chl-a was extracted by crushing the filter paper, along with the algae adhering to it, utilizing a mortar and pestle in the presence of 90% acetone. The resultant mixture was then subjected to centrifugation. In the final step, the sample containing the Chl-a extract was transferred to a cuvette, and its absorbance at wavelengths 630, 647, 664, and 750 nm was determined using a spectrophotometer. The concentration of Chl-a was computed using Eq. 1 in which L represents the circumference line on the circular filter in cm, \(\text{Vf}\) denotes the volume of filtered water in L, and \(\text{Ve}\) indicates the volume of the extract in mL (Tunali et al. 2020).
Identification of phytoplankton species
To collect phytoplankton samples at each station, 250 mL of water was also retrieved from a depth of 50 cm using sample bottles. The obtained water was then preserved in plastic containers with Lugol's solution. Following this, a sedimentation method was applied to concentrate the samples. The sample preparation process included the systematic transfer of sedimented materials from larger to progressively smaller containers, utilizing cylindrical containers with clean and thin glass bottoms. Careful attention was paid to filling the sedimentation chamber to prevent the formation of bubbles. Moreover, to prevent the dispersion of settled materials, the sample was meticulously transferred, and any excess liquid was gently decanted. After the sample underwent thorough shaking and homogenization, 5 cc of it was scrutinized and identified under an inverted microscope using a cavity slide in three replicates. Photographic documentation of the samples was conducted under the microscope after examination. Equation 2 was then utilized following the microscopic study to calculate the abundance of phytoplankton in 1 L of the specified water source, where D is the species count per L, N is the count of organisms observed in the microscopic sample (5 cc), v is the volume of concentrated water from 1 L of the sample (m3), and V is the volume of the sample observed under the microscope (m3). The species were identified using the available key identification sources (Varol and Fucikova 2015; Varol et al. 2018).
Phytoplankton diversity and dominance indices
The diversity and dominance of the phytoplankton species were investigated using four indices, Dominance Index (Y), Margalef Diversity Index (D), Shannon–Wiener Index (H′), and Pielou’s Evenness Index (J′) (Ni et al. 2018; Gogoi et al. 2019). The Dominance Index (Eq. 3) was calculated by dividing the number of individuals of the ith species (\({n}_{i}\)) by the mutiplication of total number of individuals of phytoplankton (N) and the frequency of the ith species (\({f}_{i}\)). Margalef Diversity Index was calculated using Eq. 4 where S is the total number of phytoplankton species. The proportion of the ith species in total individuals (\({P}_{i}\)) was also caculated to measure the Shannon–Wiener Index using Eq. 5. Pielou’s Evenness Index was measured by dividing the Shannon–Wiener Index by the natural logarithm of the total number of phytoplankton species (Eq. 6).
Measurement of the trophic state index
The Trophic State Index (TSI) (Carlson 1977) was utilized to assess the trophic condition of the lake. In general, TSI serves as an indicator of the nutritional equilibrium, and an elevation in nutrient concentrations induces variations in phytoplankton biomass, resulting in a decrease in water clarity and an increase in water turbidity. Accordingly, TSI offers a practical means of quantifying these associations, assigning a numerical value between 0 and 100 to each parameter based on their respective equations (Lyu et al. 2022). The parameters incorporated in this index encompass total phosphorus (TP), Chl-a, and Secchi disk depth (SD) (Eq. 7–9), evaluated using the Carlson Index.
Equation 10 serves as the total TSI, amalgamating the individual indices obtained from Eqs. 7–9. According to this index, the reservoir's condition was categorized into four states: oligotrophic (0 < TSI < 30), mesotrophic (30 < TSI < 50), eutrophic (50 < TSI < 70), and hypertrophic (70 < TSI) where oligotrophic and mesotrophic states are generally considered healthier, while eutrophic and hypertrophic states may indicate water quality issues, such as excessive nutrient loading and potential ecological imbalances (Lyu et al. 2022).
Statistical analysis
The water quality parameters measured at each station were reported as Mean ± Standard Error and underwent one-way analysis of variance (ANOVA) with Duncan LSD All-Pairwise Comparison Test to measure their statistical differences based on a p-value of 0.05. Principal Component Analysis (PCA) (Greenacre et al. 2022) was used to characterize the most important water quality parameters in each season, revealing the highest variation in the dataset. The Canonical Correspondence Analysis (CCA) (Guo and Wu 2019) was also employed to investigate the relationship between water quality parameters and the presence of phytoplankton species found in the samples. To do so, we examined the distribution of the data to identify outliers. Additionally, we assessed the normality and homoscedasticity of the data to meet the assumptions of multivariate analysis. We also ensured that the CCA variables are not highly correlated with each other, as this might cause multicollinearity (Varol and Sen 2018; Varol 2019). The statistical analysis performed in this research was perfromed using Microsoft Excel 2016 and Past 4.10.
Results
The average seasonal values for water quality parameters in Karun Lake are presented in Table 1. The highest water temperature (31.04 ± 0.60 ˚C) and pH (8.32 ± 0.06) were observed in summer. O2 and TUR were highest in spring, equaling 10.93 ± 0.23 mg/l and 50.44 ± 22.70 NTU, respectively. ALK and EC peaked in winter. The highest and lowest levels of the ions differed significantly by season. Maximum Ca2+ and Mn2+ concentrations were recorded in winter while \({\text{PO}}_{4}^{3-}\), F−, \({\text{NH}}_{4}^{+}\), and Na+ were highest in summer. The maximum SD value, measuring 3.604 m, was recorded during the winter season, while the minimum, at 1.177 m, was observed in the spring. The lowest Chl-a value in the winter season was 1.844 µg/L, and the highest, at 22.270 µg/L, was noted in the spring. The highest TP value, amounting to 74.846 µg/L, occurred in the summer season.
A total of 56 phytoplankton genera were documented and classified into the following taxonomic classes: 22 under Bacillariophyceae, 19 under Chlorophyceae, four under Dinophyceae, six under Cyanobacteria, two under Chrysophyceae, two under Charophyceae, and one under Euglenophyta (Table 2). The average annual density of phytoplankton exhibited seasonal variations, measuring 34.151 cells/ml in spring, 104.38 cells/ml in summer, 168.81 cells/ml in fall, and 11.45 cells/ml in winter. The annual frequency distribution highlighted Chrysophyceae as the predominant phytoplankton group, constituting 38%, followed by Bacillariophyceae at 32%, Chrysophyceae at 16%, Dinophyceae at 13.5%, and other genera contributing less than 0.5%. Seasonal variations revealed that 41% of the phytoplankton population was associated with summer, 26% with spring, 25% with fall, and only 8% with winter. Y index ranged between 0.136 (in spring) and 0.258 (in winter). The H′ was found to be highest in summer and lowest in winter. The D index was also greater than 0.450 in fall and winter and was lowest during summer (Fig. 2).
The mean seasonal values of the TSI parameters are presented in Fig. 3. The average TSI (SD) value ranged from 41.665 (winter) to 58.645 (spring). Similarly, the mean TSI (Chl-a) value reached its peak at 59.444 in the spring and its nadir at 32.360 in the winter. The maximum TSI (TP) value, 65.591, was observed in the summer, followed by 60.268 in the spring. According to the total TSI values, Karun Lake exhibited mesotrophic conditions during fall and winter (with values ranging between 43.506 and 47.123), and eutrophic conditions during spring and summer (with values between 53.964 and 59.453).
PCA analysis was conducted on 20 variables spanning four seasons (Table 3). The findings revealed that during the spring season, six components elucidated 80.85% of the residual variance. In spring, the initial component (PC1) encompassed parameters pH, \({\text{PO}}_{4}^{3-}\), SiO2, Na+, Mg2+, Cl−, and EC, elucidating 39.936% of the variance, with \({\text{PO}}_{4}^{3-}\) and SiO2 exhibiting negative loadings. Additionally, PC2 included Na+, Ca2+, Mg2+, \({\text{NH}}_{4}^{+}\), EC, and F− (15.403%), with Ca2+ and \({\text{NH}}_{4}^{+}\) demonstrating negative loadings. In the summer season, PCA analysis demonstrated that eight components accounted for 83.29% of the remaining variance. Its PC1 comprised Mg2+, SiO2, pH, and \({\text{NO}}_{3}^{-}\), explaining 20.315% of the variance, with pH and \({\text{NO}}_{3}^{-}\) displaying a negative correlation. PC2 included factors such as Fe2+, O2, F−, T, and TUR, with F−, TUR, and T exhibiting negative loadings. During the fall season, PCA analysis indicated that seven components explained 82.89% of the remaining variance, including Na+, Mg2+, EC, \({\text{SO}}_{4}^{2-}\), F−, \({\text{PO}}_{4}^{3-}\), and Cl− in PC1 explaining 33.71% of the variance and SiO2, Ca2+, and pH in PC2 explaining 13.117% of the variance. Results for the winter season revealed that seven components explained 78.80% of the remaining variance. The initial component included pH, \({\text{SO}}_{4}^{2-}\), Fe2+, and \({\text{SO}}_{4}^{2-}\), elucidating 21.80% of the variance, and PC2 included two factors Ca2+ and Mg2+, representing 15.32% of the variance, with Ca2+ displaying a negative correlation.
Results of CCA analysis are given in Table 4 and Fig. 4. The spring CCA showed positive loadings for Cyanobacteria, Charophyceae, and Diniphyceae groups and T, EC, Cl−, \({\text{NO}}_{3}^{-}\), Fe2+, Na+, Mg2+, and \({\text{HCO}}_{3}^{-}\) in CA1 (eigenvalue = 0.063 and total variance = 61.40%). Positive associations were also observed in summer CA1 (eigenvalue = 0.634 and total variance = 49.55%) between Diniphyceae, Chrysophyceae, Charophyceae, Euglenophyta, T, O2, EC, Cl−, \({\text{SO}}_{4}^{2-}\), F−, Ca2+, Mn2+, Na+, Mg2+, SiO2, and k. The genera of Chlorophyceae, Chrysophyceae, Euglenophyta, and Dinophyceae were positively associated with higher values in fall CA 1 (eigenvalue = 0.353 and total variance = 49.57%), suggesting that they thrive under conditions characterized by higher TUR, \({\text{SO}}_{4}^{2-}\), F−, Ca2+, Mg2+, \({\text{NH}}_{4}^{+}\), Fe2+, Mn2+, SiO2, and K. Moreover, it indicates that Bacillariophyceae, Cyanobacteria, and Charophyceae may be favored under conditions of lower T, O2, pH, TUR, ALK, EC, Cl−, \({\text{NO}}_{3}^{-}\), F−−, and Na+ in this season. In winter, Chlorophyceae, Chrysophyceae, Euglenophyta, and Dinophyceae have positive loadings on Axis 1 (eigenvalue = 0.219 and total variance = 46.96%), suggesting a positive association with the environmental variables influencing this axis including TUR, EC, \({\text{SO}}_{4}^{2-}\), \({\text{NH}}_{4}^{+}\), Fe2+, Mn2+, and SiO2.
Discussion
Water quality dynamics in Karun Lake
Dam lakes play a crucial role in providing water resources for both human use and downstream ecosystems. However, their influence can lead to changes in water quality, thus requiring water conservation and monitoring (Kakoei Dinaki et al. 2021). In Karun Lake, the pronounced peak in T during summer aligns with expectations for this season. The elevated levels of O2 and TUR in spring also point toward increased biological activity (Doda et al. 2020) and the surge in spring rainfall triggering sediment transport through runoff and surface streams during this season. However, peak levels of ALK and EC recorded in winter may be attributed to specific environmental conditions, such as geological factors or anthropogenic inputs. The seasonal variations in ion concentrations reveal a nuanced pattern, with certain ions reaching their maximum levels during specific seasons. For instance, the elevated concentrations of Ca2+ and Mn2+ in winter may be linked to geological processes or increased weathering during colder months. Conversely, the higher levels of \({\text{PO}}_{4}^{3-}\), F−, \({\text{NH}}_{4}^{+}\), and Na+ during the summer months could indicate possible impacts arising from extensive agricultural practices conducted in the area, leading to runoff entering the lake. Despite the high fluctuations in the concentration of the ions, the majority of these ions, including Ca2+, Mg2+, \({\text{NH}}_{4}^{+}\), Na+, K+, Cl−, \({\text{SO}}_{4}^{2-}\), \({\text{NO}}_{3}^{-}\), and \({\text{PO}}_{4}^{3-}\), remained within the standard limits set by the WHO (2011) for drinking purposes.
Phytoplankton dynamics and adaptability
The taxonomic groups identified in this investigation indicate a diverse phytoplankton community within the lake. The peak phytoplankton density was observed during the fall and summer seasons, while the lowest density was noted in winter. The continuous availability of sunlight and optimal temperatures during fall and summer creates favorable conditions for phytoplankton growth and development. In a similar vein, Zhang et al. (2017) suggested that warm seasons with favorable temperature and light availability induce phytoplankton productivity. This concept is further supported by Ruggieri et al. (2011) who demonstrated a positive association between solar radiation and Chl-a. Conversely, in winter, with colder water surfaces and reduced sunlight, phytoplankton experienced diminished growth, resulting in a decrease in their density. It appears that reduced biological activity and a decline in organic matter limit nutrient accessibility to phytoplankton, leading to a reduction in phytoplankton density during winter.
The yearly frequency percentage of phytoplankton indicates Chrysophyceae as the most predominant species. Typically, these algae flourish in nutrient-rich environments, and their elevated abundance can be rationalized by the heightened concentrations of \({\text{PO}}_{4}^{3-}\) and \({\text{NO}}_{3}^{-}\) in the study area. Bacillariophyceae, the second most prevalent phytoplankton group, also requires nutrient-rich substrates for their growth, and their presence can be linked to the observed escalation in ion concentrations, specifically Na+, \({\text{PO}}_{4}^{3-}\), and SiO2, within the study locale (Hernández et al. 2016; Pham 2017). Certain species within both classes exhibit resilience to lower temperatures, enabling them to thrive in cold climates (Okhapkin et al. 2022). Additionally, they have demonstrated the capacity to endure significant chemical, physical, and biological alterations, including increases in pH, elevated Na+ and \({\text{PO}}_{4}^{3-}\) levels, and a decrease in O2 levels, as evidenced in the annual characteristics of water. This adaptability underscores the robust nature of these phytoplankton classes in responding to a spectrum of environmental conditions.
Ecological responses to seasonal changes
The observed variations in dominance, richness, diversity, and evenness across seasons suggest a dynamic ecological response to seasonal changes in water properties. The Margalef Index reached its peak during the spring season, consistent with the anticipated heightened biological activity characterized by increased levels of oxygen and the rising concentration of certain ions, such as \({\text{PO}}_{4}^{3-}\). Furthermore, the persistent presence of sunlight and optimal temperatures during the fall and summer seasons, fostering favorable conditions for phytoplankton growth, contributes to the elevated measures of the Shannon–Wiener Index during these periods. As Pielou’s Evenness evaluates the distribution of abundance among species, the decreased biological activity, and declines in organic matter during winter restrict nutrient accessibility to phytoplankton, resulting in an even distribution of phytoplankton species in this season. Comparable findings have been reported in prior research, such as the study by Ding et al. (2021) which demonstrated that the phytoplankton community in surface water during the summer is significantly more diverse and less even than in the winter. This difference is attributed to factors such as sunlight availability, favorable temperatures, and the proliferation of ions, influencing the overall diversity and evenness of the phytoplankton community. Overall, peaks in the Margalef Index are expected to align with seasons of increased biological activity and optimal conditions for growth, while fluctuations in the Shannon–Wiener Index and decreases in Pielou’s Evenness are seemingly associated with seasonal variations in nutrient availability and phytoplankton growth.
Results showed that TP levels peaked in summer, indicating a potential influx of nutrients during this season. Increased TP may contribute to higher algal growth. Moreover, lower Chl-a levels in winter might be due to reduced biological activity and limited sunlight, leading to decreased photosynthesis and algal growth. Furthermore, factors such as reduced algal growth or sedimentation during the colder seasons increased SD from spring to winter. Similar to the trends observed in TP, Chl-a, and SD, TSI values followed a similar pattern, with the highest values observed in spring. These results reflect the eutrophic condition in spring and summer, transitioning to mesotrophic conditions in fall and winter, which is probably due to seasonal variations observed in nutrient availability and biological activity. It seems that the seasonal shifts in the lake's trophic status are influenced by factors such as T, light availability, and nutrient inputs where spring and summer exhibit higher nutrient levels and biological activity, leading to eutrophic conditions. In contrast, fall and winter demonstrate a decline in these parameters, resulting in mesotrophic conditions.
Multivariate analysis: PCA and CCA insights
The PCA test results indicate distinctive patterns of variable contributions to the principal components in each season. In spring and winter, PC1 displays robust positive loadings for pH, signifying a positive correlation with the overall variability in the dataset. This suggests that variations in pH significantly influence the observed changes in water quality during spring and winter. In summer, the PCA results suggest a potential impact of nutrient concentrations and pH on the observed variability. Furthermore, PCA outcomes during this season highlight the influence of parameters related to water oxidation and turbidity, with positive loadings for Fe2+, and O2, and negative loadings for F−, TUR, and T. Overall, commonalities observed across seasons include the notable influence of pH, major ions (Na+ and Mg2+), and nutrient concentrations (\({\text{PO}}_{4}^{3-}\) and SiO2) in shaping water quality.
CCA was employed to unravel the complex interplay between environmental variables and aquatic species composition in the surface waters of Karun Lake. The findings revealed that Bacillariophyceae species exhibit optimal growth in waters with elevated ionic content during spring and summer, a characteristic often associated with increased runoff and nutrient availability during the spring season. Notably, these species were found to be less abundant in alkaline and turbid conditions during these seasons. Additionally, in winter, their presence was diminished in environments characterized by higher bicarbonate and alkalinity levels. Ács et al. (2019) also found that the diversity of Bacillariophyceae species decreases notably in ponds characterized by turbidity and higher alkalinity. Similarly and in agreement with the results of Smucker et al. (2021), Cyanobacteria exhibited a strong positive association with temperature, indicating a preference for warmer temperatures during both spring and winter.
During summer, Cyanobacteria thrived in nutrient-rich conditions, showcasing positive associations with Mg2+, SIO2, and K+. Furthermore, Cyanobacteria were consistently found to favor well-oxygenated environments and areas with higher iron concentrations across all seasons. Chlorophyceae, on the other hand, displayed positive associations with O2, pH, and \({\text{NO}}_{3}^{-}\), indicating a preference for well-oxygenated and slightly alkaline waters with nitrate availability, particularly in spring. This group also demonstrated a propensity for nutrient-rich conditions throughout the year, as evidenced by positive associations with Mg2+, \({\text{NH}}_{4}^{+}\), Na2+, and K+. Despite the shared affinity for nutrient-rich conditions, the specific preferences for individual nutrients and ions varied by season and species groups. This variability underscores the dynamic nature of the water environment in the region, influencing the presence and abundance of phytoplankton species. Notably, water temperature emerged as a crucial factor in spring and summer, while lower favorability was observed in environments with higher bicarbonate and alkalinity levels during winter and fall. These consistent patterns across species and seasons underscore the importance of understanding the interplay between environmental variables and phytoplankton dynamics in Karun Lake.
Conservation implications
The distinct patterns observed across species and seasons underscore the dynamic nature of the water environment in Karun Lake, emphasizing the need for adaptive management strategies that consider seasonal fluctuations in environmental conditions. Particularly, spring and summer were found to harbor a nutrient-rich environment, indicative of increased runoff and nutrient availability during these seasons, thereby facilitating the proliferation of certain species such as Bacillariophyceae. Additionally, seasons characterized by reduced alkaline and turbid conditions are prevalent in the region, resulting in diminished abundance of sensitive species and highlighting the necessity of maintaining water quality to support diverse phytoplankton communities. Consequently, adopting adaptive management approaches that account for temporal fluctuations in environmental conditions emerges as a more effective strategy for this region. Specifically, during summer, more stringent strategies aimed at mitigating nutrient inputs and managing algal blooms may be warranted to uphold ecosystem health and water quality in Karun-4 Lake.
Limitations and suggestions
The study primarily focused on surface water, limiting the depth of insights into potential vertical variations within the lake. A more comprehensive analysis of water layers could offer a nuanced understanding of the lake's dynamics and ecological interactions. Additionally, the research did not consider the potential impact of extreme weather events, which can play a pivotal role in shaping the lake's ecosystem. Future investigations should prioritize incorporating these factors to provide a more holistic perspective and strengthen the overall robustness of the findings. Moreover, it is crucial to assess the ubiquitous effect of human pressures and pollution on lake aquatic species, as substantially demonstrated in Iranian ecosystems (Nourouzi et al. 2018). To address these limitations, it is crucial to implement water conservation measures and establish ongoing monitoring programs for Karun Lake. Specifically, the adoption of adaptive management strategies is recommended, taking into account the dynamic nature of the ecosystem, particularly during critical seasons. By doing so, we can enhance our ability to respond effectively to environmental changes and contribute to the sustainable management and preservation of Karun Lake.
Conclusion
This study provides valuable insights into the complex interplay of factors influencing water quality, phytoplankton dynamics, and ecological responses in Karun Lake. The evident variations in water parameters underscore the lake's vulnerability to environmental shifts, emphasizing the impact of temperature, sunlight, and nutrient influx. The adaptability of the phytoplankton community, particularly the prevalence of Chrysophyceae (38%) and Bacillariophyceae (32%), highlights the lake's nutrient-rich conditions, particularly during the spring season. Ecological indices also mirrored the lake's dynamic nature, with heightened biological activity during spring (TSI total = 59.435) and summer (TSI total = 53.964). However, the study emphasizes the need for more in-depth analyses of vertical variations and the consideration of extreme weather events in the future investigations. To address these gaps, the conclusion advocates for adaptive management strategies, ongoing monitoring initiatives, and water conservation measures tailored to the dynamic ecosystem of Karun Lake.
Data availability
The datasets applied and/or analyzed throughout the present research are available from the corresponding authors on reasonable request.
References
Abd Ellah RG (2020) Water resources in Egypt and their challenges Lake Nasser case study. Egypt J Aquat Res. https://doi.org/10.1016/j.ejar.2020.03.001
Ács É, Földi A, Vad CF, Trábert Z, Kiss KT, Duleba M, Borics G, Grigorszky I, Botta-Dukát Z (2019) Trait-based community assembly of epiphytic diatoms in saline astatic ponds: a test of the stress-dominance hypothesis. Sci Rep-UK. https://doi.org/10.1038/s41598-019-52304-4
Asadian N, ChamaniAbolhasani AMH (2020) The trophic status of the Zayandeh River dam lake in the spring and summer. Int J Aquat Biol 8(3):209–215
Ashjari J, Soltani F, Rezai M (2019) Prediction of groundwater seepage caused by unclogging of fractures and grout curtain dimensions changes via numerical double-porosity model in the Karun IV River Basin (Iran). Environ Earth Sci. https://doi.org/10.1007/s12665-019-8054-1
Bao L, Chen J, Tong H, Qian J, Li X (2022) Phytoplankton dynamics and implications for eutrophication management in an urban river with a series of rubber dams. Environ Manage. https://doi.org/10.1016/j.jenvman.2022.114865
Buta B, Wiatkowski M, Gruss L, Tomczyk P, Kasperek R (2023) Spatio-temporal evolution of eutrophication and water quality in the Turawa dam reservoir. Sci Rep-UK, Poland. https://doi.org/10.1038/s41598-023-36936-1
Carlson RE (1977) A trophic state index for lakes 1. Limnol Oceanogr. https://doi.org/10.4319/lo.1977.22.2.0361
Ding C, Sun J, Narale DD, Liu H (2021) Phytoplankton Community in the Western South China Sea in winter and summer. Water. https://doi.org/10.3390/w13091209
Doda T, Ramón CL, Ulloa HN, Brennwald MS, Kipfer R, Schubert C, et al (2020) Lateral transport of dissolved gases by cooling-driven density currents in a small temperate lake. In 18th Swiss Geoscience Meeting, Zurich
Gecheva GM, Belkinova DS, Varadinova ED (2020) Phytoplankton, macrophytes and macroinvertebrates in reservoirs: response to eutrophication. Ecol Balk 12(2):153–164
Glibert PM (2017) Eutrophication, harmful algae and biodiversity—challenging paradigms in a world of complex nutrient changes. Mar Pollut Bull. https://doi.org/10.1016/j.marpolbul.2017.04.027
Gogoi P, Sinha A, Das Sarkar S, Chanu TN, Yadav AK, Koushlesh SK, Borah S, Das SK, Das BK (2019) Seasonal influence of physicochemical parameters on phytoplankton diversity and assemblage pattern in Kailash Khal, a tropical wetland, Sundarbans, India. Appl Water Sci. https://doi.org/10.1007/s13201-019-1034-5
Greenacre M, Groenen PJ, Hastie T, d’Enza AI, Markos A, Tuzhilina E (2022) Principal component analysis. Nat Rev Methods Primers. https://doi.org/10.1038/s43586-022-00184-w
Guo C, Wu D (2019) Canonical correlation analysis (CCA) based multi-view learning: an overview. arXiv preprint arXiv:1907.01693. https://doi.org/10.48550/arXiv.1907.01693.
Hernández KL, Yannicelli B, Olsen LM, Dorador C, Menschel EJ, Molina V, Remonsellez F, Hengst MB, Jeffrey WH (2016) Microbial activity response to solar radiation across contrasting environmental conditions in Salar de Huasco, Northern Chilean Altiplano. Front Microbiol. https://doi.org/10.3389/fmicb.2016.01857
Kakoei Dinaki F, Cheraghi M, Lorestani B, SobhanArdakani S, Chamani A (2021) Investigating the potential of native plants in phytoremediation of heavy metals and nutrients in the latian and lar dam reservoirs. Water Resour Res 17(2):130–141 (in Persian)
Kazemi M, Chamani A, Agh N (2019) The assessment of arsenic contamination in urmia lake sediments and its effect on human health. J Environ Sci Stud 45(3):485–497
Li Y, Ni L, Guo Y, Zhao X, Dong Y, Cheng Y (2022) Challenges and opportunities to treat water pollution. Paths Clean Water under Rapid Chang Environ China. https://doi.org/10.1007/978-981-19-0091-4_2
Lyu L, Song K, Wen Z, Liu G, Shang Y, Li S, Tao H, Wang X, Hou J (2022) Estimation of the lake trophic state index (TSI) using hyperspectral remote sensing in Northeast China. Opt Express. https://doi.org/10.1364/OE.453404
Moshir Panahi D, Kalantari Z, Ghajarnia N, Seifollahi-Aghmiuni S, Destouni G (2020) Variability and change in the hydro-climate and water resources of Iran over a recent 30 year period. Sci Rep-UK. https://doi.org/10.1038/s41598-020-64089-y
Ni M, Yuan JL, Liu M, Gu ZM (2018) Assessment of water quality and phytoplankton community of Limpenaeus vannamei pond in intertidal zone of Hangzhou Bay, China. Aquaculture Rep. https://doi.org/10.1016/j.aqrep.2018.06.002
Nourouzi MM, Chamani A, Shirani M (2018) Effect of Cd and Pb pollutions on physiological growth: wavelet neural network (WNN) as a new approach on age determination of coenobita scaevola. Bull Environ Contam Toxicol 101:320–325
Okhapkin A, Vodeneeva E, Sharagina E, Kulizin P (2022) Composition and structure of phytoplankton of Lake Svetloyar (Russia). Inland Water Biol. https://doi.org/10.1134/S1995082922050169
Patale VV, Tank JG (2022) Evaluation of the edaphic and water properties of Diu coast (Saurashtra, Gujarat, India) in relation to the population density of Avicennia marina. Appl Water Sci. https://doi.org/10.1007/s13201-022-01602-w
Pham TL (2017) Environmental gradients regulate the spatio-temporal variability of phytoplankton assemblages in the Can Gio mangrove biosphere reserve, Vietnam. Ocean Sci J. https://doi.org/10.1007/s12601-017-0045-0
Rice EW, Bridgewater L, Association APH (2012) Standard methods for the examination of water and wastewater, vol 10. American public health association Washington, DC
Ruggieri N, Castellano M, Capello M, Maggi S, Povero P (2011) Seasonal and spatial variability of water quality parameters in the Port of Genoa, Italy, from 2000 to 2007. Mar Pollut Bull. https://doi.org/10.1016/j.marpolbul.2010.10.006
Salman SA, Shahid S, Sharafati A, Salem GSA, Bakar AA, Farooque AA, Chung ES, Ahmed YA, Mikhail B, Yaseen ZM (2021) Projection of agricultural water stress for climate change scenarios: a regional case study of Iraq. Agriculture. https://doi.org/10.3390/agriculture11121288
Saturday A, Lyimo TJ, Machiwa J, Pamba S (2023) Spatial and temporal variations of trophic state conditions of Lake Bunyonyi, south-western Uganda. Appl Water Sci. https://doi.org/10.1007/s13201-022-01816-y
Shahradnia H, Chamani A, Zamanpoore M (2021) Heavy metal pollution in surface sediments of Ghareh-Aghaj River, one of the longest perennial rivers in Iran. Environ Earth Sci 80:91–102
Shahradnia H, Chamani A, Zamanpoore M (2022) Linking river sediment arsenic to catchment spatial attributes in agricultural landscapes. Environ Sci Pollut Res 29:2830–2838
Singh P, Bagrania J, Haritash A (2019) Seasonal behaviour of thermal stratification and trophic status in a sub-tropical Palustrine water body. Appl Water Sci. https://doi.org/10.1007/s13201-019-1011-z
Smucker NJ, Beaulieu JJ, Nietch CT, Young JL (2021) Increasingly severe cyanobacterial blooms and deep water hypoxia coincide with warming water temperatures in reservoirs. Glob Change Biol. https://doi.org/10.1111/gcb.15618
Statistical Center of Iran S (2016) Khuzestan statistical year book, Tehran, Iran
Tunali M, Uzoefuna EN, Tunali MM, Yenigun O (2020) Effect of microplastics and microplastic-metal combinations on growth and chlorophyll a concentration of Chlorella vulgaris. Sci Total Environ. https://doi.org/10.1016/j.scitotenv.2020.140479
Varol M (2019) Phytoplankton functional groups in a monomictic reservoir: seasonal succession, ecological preferences, and relationships with environmental variables. Environ Sci Pollut Res 26(20):20439–20453. https://doi.org/10.1007/s11356-019-05354-0
Varol M, Şen B (2018) Abiotic factors controlling the seasonal and spatial patterns of phytoplankton community in the Tigris River. Turkey River Res Appl 34(1):13–23. https://doi.org/10.1002/rra.3223
Varol M, Blanco S, Alpaslan K, Karakaya G (2018) New records and rare taxa for the freshwater algae of Turkeyfrom the Tatar Dam Reservoir (Elazığ). Turk J Bot 42(4):533–542. https://doi.org/10.3906/bot-1710-55
Varol M, Fucikova K (2015) Four new records for the freshwater algae of Turkey. J Limnol Freshwater Fish Res 1(2):83–88. https://doi.org/10.17216/LimnoFish-5000119624
Wang S, Li J, Zhang B, Spyrakos E, Tyler AN, Shen Q et al (2018) Trophic state assessment of global inland waters using a MODIS-derived Forel-Ule index. Remote Sens Environ. https://doi.org/10.1016/j.rse.2018.08.026
Wassie TA, Melese AW (2017) Impact of physicochemical parameters on phytoplankton compositions and abundances in Selameko Manmade Reservoir, Debre Tabor, South Gondar, Ethiopia. Appl Water Sci. https://doi.org/10.1007/s13201-015-0352-5
WHO (2011) Guidelines for drinking-water quality. WHO Chronicle 38(4):104–108
Zhang C, Zhang W, Huang Y, Gao X (2017) Analysing the correlations of long-term seasonal water quality parameters, suspended solids and total dissolved solids in a shallow reservoir with meteorological factors. Environ Sci Pollut Res. https://doi.org/10.1007/s11356-017-8402-1
Funding
The author received no specific funding for this work.
Author information
Authors and Affiliations
Contributions
Data collection was performed by Nader Cheraghpour-Ahmadmahmoodi, Mohsen Saadat, Rasool Zamani-Ahmadmahmoodi, and Avid Avokh. Nader Cheraghpour-Ahmadmahmoodi, Mohsen Saadat, and Rasool Zamani-Ahmadmahmoodi supervised the research and designed experiments and analyzed data and co-wrote the paper.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval and consent to participate
Not applicable.
Consent for publication
Not applicable to this study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Cheraghpour-Ahmadmahmoodi, N., Saadat, M., Zamani-Ahmadmahmoodi, R. et al. A four-season exploration of surface water quality and trophic status in the highly dynamic waters of Karun-4 Dam Lake, SW Iran. Appl Water Sci 14, 158 (2024). https://doi.org/10.1007/s13201-024-02222-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13201-024-02222-2