Abstract
Egypt is a country that heavily relies on agricultural activities. River Nile serves as the primary source of irrigation water for agricultural purposes. El Saieda Basin project, located in the southern western desert of Egypt, represents a massive agricultural project where irrigation water is distributed from the River Nile through an extensive network of canals. This study focuses on evaluating the groundwater resources, the groundwater quality, and the hydrochemical processes affecting the groundwater within El Saieda Basin to provide an additional source of irrigation water and to ensure a safe water supply for the residential villages. Various data sets were integrated, including geomorphology, geology, hydrogeology, hydrogeochemistry and stable isotopes. Based on the geomorphology; the study area includes four geomorphological units, Structural Plateau, Bajada Plain, Eroded old Nubian surface, and Nile alluvial Plain. According to the geology; the study area is located in the southern part of the Western Desert. The dominating geological units in this part of Egypt range in age from Lower Cretaceous to Recent. Regarding the hydrogeology of the study area; the Quaternary aquifer is the main aquifer in the study area where 24 groundwater samples plus two surface water samples (irrigation canal + main Drain) were collected and analyzed. Groundwater salinity classification shows that 17% of the total groundwater samples are fresh groundwater samples with values that range between 550 and 996 mg/l with an average of 775 mg/l. 29% of the total groundwater samples are brackish groundwater samples with values that range between 1177 and 2273 mg/l with an average of 1868 mg/l. In addition, 46% of the total groundwater samples are salty groundwater samples with values that range between 3197 and 4886 mg/l with an average of 4037 mg/l. The rest of the groundwater samples (8%) are saline groundwater samples with a maximum value of 9018 mg/l and an average of 7645 mg/l. The groundwater's temperature ranges from 25 to 30.9 C. However, the measured pH values showed relatively neutral water where the values range from 6.6 to 7.6. The relationship between salinity content and the other ions indicates that sodium, calcium, magnesium, sulfate, and chloride are the effective ions that increase the salinity content. Silicate weathering and dissolution of calcite are the main hydrochemical processes affecting the groundwater. From the saturation indices (SI) results it is indicated that Gypsum and Anhydrite dissolve with negative SI values, whereas iron minerals are supersaturated with positive values. Carbonate minerals are insufficiently soluble in groundwater, with values that fluctuate positively and negatively around zero. From the hypothetical salts, it is indicated that the quaternary groundwater aquifer in the study area is highly evolved as it's enriched with terrestrial and marine residuals indicated by the presence of NaCl, MgCl2, MgSO4, CaSO4, Ca(HCO3)2 salts. From the Environmental Stable Isotopes, it is indicated that all the groundwater samples are enriched by 18O and Deuterium which indicates a recharge from the main irrigation canal and return flow irrigation water. Trace elements results indicated that the groundwater is suitable for human drinking according to the World Health Organization and also for irrigation according to the Australian guidelines for irrigation water quality.
Similar content being viewed by others
Explore related subjects
Find the latest articles, discoveries, and news in related topics.Avoid common mistakes on your manuscript.
Introduction
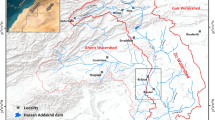
Egypt has a total area of over 1 million km2 (about 1,019,600 km2), (Said 1962; Abd El-Ghani et al. 2017), which is desert lands except a small cultivated area restricted in the Nile Valley and delta, occupying 4% of Egypt area. Owing to the limited natural resources, and the alarming rate of over growing population, horizontal expansion of agricultural land has to eliminate this problem and narrow the gap that exists between food production and consumption, (Awad et al. 2020). Therefore, to find an excellent solution to overcome these problems, the Egyptian authorities' strategy was adopted to cope with this situation to expand and extend land productivity in horizontal and vertical aspects. In this accord, tremendous efforts and huge investments are underway, with the assistance of national and international agencies and organizations, by bringing new lands under cultivation. Among the areas suitable for such agricultural expansion in the western desert is the El-Saieda Basin due to its water and land potentialities. El-Saieda Basin is located in the Western Desert. It belongs to Aswan Governorate, 100 km in the North, and is located in the northwestern part of Edfu city. The investigated area contains six villages, Al Shahama, Amr Ebin Al As, Al Eman, Al Smaha, Al Ashraf, and Al Nemo, (Sweed et al. 2023). It is bounded by longitudes 32° 10′ 00″ and 32° 55′ 00″ E and latitudes 24° 50′ 00″ and 25° 10′ 00″ N, covering a surface area of approximately 450,000 Acres. The area is bounded on the northern side by El-Besaliya village, on the southern side by El Galaba plain, on the eastern side by Idfu and Nile River, and on the western side by Sin El-Kaddab plateau (Fig. 1). Evaluating the groundwater resources and their controlled structures demands scientific techniques. As a result, the research aims to study the hydrogeochemical processes and evaluate the groundwater quality to develop the area under investigation this is because the development depends on the quality and the sustainability of the groundwater in the arid regions and any investment in the study area will depend on the suitability of the groundwater for the different uses and the hydrogeochemical processes which induce in the groundwater and its conditions so the results of the study will give the confidence to the decision maker. The climatic data was averaged from the past 30 years (1992 to 2021), which is the average maximum temperature (27.88 to 45.27) and the average minimum temperature (3.3 to 23.66). The mean precipitation rate was less than 1 mm, (Sweed et al. 2023).
Geomorphology
The geomorphic units were recognized and delineated by analyzing the main landscape extracted from Landsat 7ETM + satellite image, with the aid of a geomorphological map of Aswan by UNDP/UNESCO/NARSS/EGSMA (2002) and field investigations, with the aid of Shata, (2008). The obtained results included the main geomorphic units and subunits of the studied area from west to east, (Fig. 2), which are: The Structural Plateau, Bajada Plain, Eroded old Nubian surface (Peneplain), and Nile alluvial Plain.
The first geomorphologic unit is the Structural plateau which occupies the western portion of the study area and rises to more than 400 m above sea level. The surface of the plateau is covered by hard limestone. This plateau is bounded on the eastern side by a precipitous escarpment facing the Nile valley. The escarpment face is dissected by several Basins which are directed eastward to the Nile Valley. The channels of such Basins are almost covered by young deposits, (Mohamed and Sylvain 2007). The second geomorphologic unit is the Bajada plain which starts from the foot slopes of the escarpment face and is differentiated into two types, a higher colluvial plain, and a lower pavement plain; the higher colluvial plain is composed of coarse-textured driven from the plateau and the escarpment. Its elevation ranges from 268 to 335 m above sea level. The surface is covered locally by shifting sand. The lower pavement plain is composed of medium textured material, also driven from the plateau. Its elevation ranges from 210 to 273 m above sea level. The surface is also in part covered by shifting sand, (Bach, 2008). On the other hand, The Eroded old Nubian surface, (Peneplain) is considered from the main geomorphologic units where the concept of a peneplain is meaning almost a plain. It is formed by the action of water and/or wind erosion. Formation of peneplain is a result of eroding the upper parts of the surface and then the hard rocks generally appear above it, (Planation Surface 2008). This surface occupies much of the elevated plains bordering the Nile Valley. It is underlain by dank brown sandstone beds belonging to the Nubian sequence. The surface is strongly eroded by wind and is covered by shifting sand. Its elevation is about 288 m above sea level. This surface forms local cliffs overlooking the Nile floodplain. The last main geomorphologic unit in the study area is the Nile alluvial plain which is distinguished in the old Nile terraces and the present Nile floodplain the old one occupies a wide area to the west of the present Nile flood plain. The surface of the old Nile terraces rising to 196 m above sea level slopes from west to east. The present Nile flood plain occupies the lowest portion of the study area and is covered by Nile silt deposits. Its surface rises to 95 m above sea level.
Geology
According to Said, (1962), the study area is located on the Nubian sandstone, which was the subject of the study by many workers such as; Shukri and Ayouty (1953), Little and Attia (1943). The studied area is characterized by a recent group of River terraces. These terraces have a decreasing elevation towards the Nile River and they are covered throughout with fluvial sediments which were deposited at the time of formation of Nile terraces. These sediments belong to the late Tertiary and Early Quaternary. The Nubian sandstone formation includes subordinate layers of limestone and it could belong to the upper Cretaceous. In addition, it is still covered with a thin cap of hard lower Eocene limestone.
The geological map of Aswan (Fig. 3) published by the United States Agency for International Development, USAID (1978), reveals the following succession from top to bottom, (Table 1). Generally, the study area locates in the southern part of the western Desert of Egypt. The dominating rock units in this part of Egypt range in age from the Lower Cretaceous to Recent.
Geological map of the studied area, after USAID 1978
The Cenozoic Era is represented by rocks and deposits belonging to the Quaternary and the Tertiary in addition the Mesozoic Era is represented by rocks and deposits belonging to the Cretaceous. The Quaternary and the Tertiary deposits dominate the study area. Among the Quaternary, Pleistocene deposits are exposed in the study area, while the Tertiary is represented by Pliocene, lower Eocene, and Paleocene. The lithostratigraphic units and formations in the study area are discussed from top to base (from the Quaternary to the Lower Cretaceous succession) as follows:
1. The quaternary which contains the following deposits
(A) Pleistocene deposits which are considered the older Nile deposits adjacent to the Nile valley and it composed of quartz sand and gravel which have an exposed thickness of about 32 m (Qena formation). It has a maximum reported thickness of about 120 m and forms the main Quaternary aquifer in Nile valley, (Said 1981).
(b) Pliocene deposits, (marine deposits thickness > 50 m) it is essentially recorded in the subsurface. It overlies the eroded surface of the Eocene carbonates and acts as an aquiclude for the overlaying Quaternary aquifer.
2.The tertiary which contains the following deposits
(A) Lower Eocene deposits, this rock unit is made up of karstified chalky and dolomitic limestone and marl with flint bands nodules, (Thebes formation). It is best exposed in the westward direction with more than 500 m. The thickness of Eocene carbonate in the subsurface attains a total thickness of about 2000 m and is unconformably underlain by the basement rocks. It is generally made up of carbonates which alter to sandstone in its lower parts.
(b) Paleocene deposits are dominated by shale faces with thin interbeds of chalk and phosphate (Dakhla formation). It acts, generally, as an aquiclude separating the Eocene fissured carbonate aquifer from the Nubian aquifers. It occurred at the latitude of Idfu. This unit has a thickness of about 400 m.
3.The cretaceous
Cretaceous, this unit could be classified into lower and upper Cretaceous. The upper Cretaceous deposits are white chalk, chalk limestone, and dolomite, while the lower Cretaceous is composed of Nubian sandstone and has an exposed thickness of about 300 m. In the subsurface, the thickness is expected to increase to more than 1000 m.
Hydrogeological conditions
There are two main sources for irrigation in the study area, as follows:
Main irrigation canal
The main irrigation canal gets its water directly from the Nile River through five lifting stations to pump about 75 thousand cubic meters, (Elwan 2008) of water through four canals to irrigate the agricultural fields, but this amount of water is not sufficient for the demands of the irrigation which forced farmers to dig wells to meet their water needs.
Groundwater
Groundwater conditions in El-Saieda Basin were discussed depending on the data of 24 wells tapping into the Quaternary aquifer. Abd El-Kreem (2000) studied the origin of this water and found that its water supply comes from the Quaternary aquifer which is considered semi-confined and occupies the central part of the study area. Identification of this aquifer is made with the help of the geophysical survey results and well drilling. The aquifer consists of Pleistocene-graded sand and gravel with clay intercalations. Its thickness ranges between 120 m in the central portion to 40 m in the borders. This aquifer discharged by the extraction through wells for the irrigation and drinking purposes. The depth to the groundwater surface ranges from 2 to 28 m.
Nubian Sandstone aquifer occupies the southeastern portion of the study area. This aquifer has wide distribution in the desert and southern part of Egypt. Its thickness is about 100 m with an exposed thickness of about 300 m. Previous studies and investigation indicated that the groundwater is fossil water with ages varying from 20,000 to 40.000 years, (Elwan 2008). The discharge area of this aquifer is mainly restricted to the fault planes or through leakage to Upper hydrogeological units under high pressure. It is considered the most promising aquifer system for the future development in the Egyptian water resources plan. Nubian Sandstone aquifer is not represented in the study area by any samples.
Materials and methods
In November 2020, water samples were collected from 26 sites (24 samples from the quaternary groundwater aquifer + 2 surface water samples) under natural flow conditions. (during the high flow season). Sampling sites include hand dug wells, drilled wells (Open Holes without any design), the main irrigation canal, and the main El Saieda Basin Drain. The depth to the groundwater surface was measured by the Sounder. The total depth for each sample was determined by the Owner, (Table 2). The collected boreholes represent the only productive wells in the study area, according to our knowledge and field investigations. Samples were collected after wells were purged briefly to ensure a representative sample. The geographical locations (expressed in latitude and longitude) of the sample points, located in the field with a Global Positioning System (GPS) device are displayed in Fig. 4. The temperature, pH, and electrical conductivity (EC) were measured directly on-site using portable meters (JENWAY Model 430). At each location, three sample bottles were collected. Each bottle was rinsed three times with sample water before sampling. One bottle was collected without headspace for anions analysis. The second bottle was collected and filtered on-site using 0.45 micron filters and acidified with concentrated nitric acid (HNO3) for the preservation of dissolved metals and cations were analyzed from this bottle. The third bottle was collected for stable isotopes of oxygen and hydrogen analysis. This bottle was collected without filtration, with tight closures, and without any headspace to avoid the possibility of sample evaporation, which can affect the isotope results. The water samples were properly preserved in cold storage until the analyses were performed. The groundwater analyses were performed in the Hydrogeochemistry Department, Desert Research Center (DRC), Egypt, according to the methods adopted by the American Society for Testing and Materials (ASTM 2002). Total Dissolved Solids (TDS) were measured by the evaporation method, Calcium (Ca) and magnesium (Mg) were attempted titrimetrically using standard Na2-EDTA, Chloride (Cl) by standard AgNO3 titration, Carbonate (CO3) and bicarbonate (HCO3) by titration with H2SO4, sodium (Na) and potassium (K) by flame photometry, sulfate (SO4), is determined by the turbidity method using UV/Visible spectrophotometer, Unicam model UV4-200 (UK); wavelength 420 nm. The concentrations of the analyzed chemical constituents are reported in milligrams per liter (mg/l). As required, a milliequivalent per liter (meq/l) concentration is calculated. Charge balance error (CEB) is calculated as the difference between the total cations and anions, normalized to the total cations and anions (expressed as millequivalents/l). The percent charge-balance error is usually expressed as (Eq. 1) According to Freeze and Cherry (1979) and Kehew (2001).
If the CBE is less than 5%, the analysis is considered acceptable. The charge balance errors observed in the studied samples range from −2.57 to + 2.45, with an average of + 0.41, (Table 3). Concentrations of heavy metals ions (Ag, Al, B, Ba, Cd, Cr, Cu, Fe, Li, Mn, Mo, Ni, Pb, Sr, and Zn) in all collected samples were done using Inductively Coupled Argon Plasma, ICAP 6500 Duo, Thermo Scientific, England standard solution of 1000 mg/L, Merck, Germany were used as stock solutions for instrument standardization. The results were tabulated in mg/l, (Table 3) for interpretations. Moreover, a Picarro L2130-I isotope analyzer was used for the performance of stable isotopes analysis such as O-18 and deuterium for samples Nos. 1, 3, 5, 12, 15, 20, 22, 23, 27, Nile River, Irrigation canal and the main agriculture Drain in the laboratory of the Desert Research Center, Egypt. Precision for δ18O/δD measurement with a liquid sample is guaranteed to be 0.025/0.1, and drift over 24 h period is guaranteed to be 0.2/0.8.
The groundwater samples' chemical data was interpreted using a variety of techniques, including graphs, ion correlations, statistical analysis, software, and geochemical modeling. Piper plot (Piper 1944) was used to identify the various chemical types of the studied groundwater samples. The primary hydrochemical facies of the numerous operations that govern groundwater chemistry could also be illustrated by plotting the constituents of the analyzed water samples on the Chadha diagram, (Chadha 1999). Hydrochemical coefficients (ion ratios) are correlations between the components of the dissolved groundwater that show the stoichiometric balance of the different ions and suggest the plausible hydrochemical processes affecting the solution. These ratios are useful for determining the chemical changes that significantly affect the quality of the groundwater such as leaching and dissolution, contamination, mixing, and ion exchange. The Ion coefficients that are of certain importance in this respect are Na/Cl, Ca/Mg, and SO4/Cl. Hypothetical salts Assemblages were discussed according to Collin, (1923).
The hydrochemical program PHREEQC is used to perform the mass-balance simulation, which shows chemical reactions and changes in chemical nature, such as the dissolution/precipitation of minerals and gases in the groundwater flow channel. The groundwater system's ability for minerals to dissolve or precipitate is demonstrated by the results of Saturation-Index (SI) values (Parkhurst and Appelo 2013). The following equation would be used to calculate the saturation indices for the minerals that should exit or enter the solution: SI is equal to log (IAP/Kt), where Kt is the equilibrium solubility constant of the mineral and IAP is the ion-activity product in a diffused charged species solution. A negative value on the SI for any mineral phase means that the water is under saturated regarding that mineral and will disintegrate until equilibrium is attained, whilst a positive sign means that the water is oversaturated concerning that mineral and the mineral will depart the solution or be precipitated.
SPSS, or Statistical Package, is a statistical program that can be used to analyze any type of scientific data to determine the pattern of variation among variables or compress data into a manageable number of components or variables. The hydrochemical parameters (such as TDS, Ca, Mg, Na, HCO3, SO4, and Cl−) are among the variables used to identify the hydrogeochemical processes and the mineralization of the groundwater. 18O/D, a stable isotope that occurs naturally, was used to delineate the recharge sources of the groundwater. (Table 4)
Results and discussions
Hydrochemistry
From the hydrochemical analysis results, (Table 3), it is clear that the values of standard deviation show a lot of differences (5.5 to 1937.6) among chemical variables. The wide distribution of chemical variables in the groundwater system as a result of the influence of numerous geochemical processes occurring in the research area may be the source of this discrepancy (Subba Rao et al. 2017). At the same time, there are no wide differences in the values of the coefficient of variation (61 to 80%). This reflects the influence of only one source of the chemical variables in the aquifer system (Subba Rao and Chaudhary 2019). Concentrations of TDS, Ca2+, Mg2+, Na+, K+, HCO3−, SO4−2, and C1− exceed the drinking water quality standard limits of 500, 75, 30, 200, 12, 300, 200, and 250 mg/l in 100%, 83%, 71%, 88%, 29%, 37%, 87% and 83% of the total groundwater samples, respectively (Table 5), causing health implications (WHO 2017).
Physico-chemical data
Table 3 lists the physico-chemical characteristics of the examined samples (temperature and pH). The groundwater's temperature ranges from 25 to 30.9° C. The temperature of the groundwater samples should be measured directly at the outflow because it may be equilibrated with the ambient temperature during the sampling (Huneau et al. 2011). The reported pH values measured at the wellhead are between 6.6 and 7.6 in the groundwater. The pH value recorded for the irrigation canal and the main drain of the study area is 7.5. From the results, the pH of the groundwater and the surface water in the study area is considered neutral water where the pH ranges from 6.6 to 7.6.
Total dissolved solids (TDS)
Values of the total dissolved solids (TDS) in the groundwater are highly variable ranging from 550 to 9018 mg/l for the groundwater samples. The large increase in salinity is mainly attributed to the tectonic activity and the facies changes in geological formations, (Farid et al. 2015). In general, the salinity of the surface water belongs to fresh water type, where the salinity contents of the main irrigation canal and the main drain in the study area are 361.39 and 703.78 mg/l respectively, (Table 3).
Groundwater samples can be classified into three groups based mainly on the TDS contents (Gattacceca et al. 2009). Fresh groundwater which has TDS values less than 1000 mg/l represented about 17% of the total groundwater samples. Brackish water (TDS = 1000–3000 mg/l), represented 29% of the total groundwater samples which characterizes samples Nos. 5, 8, 12, 18, 19, 20, and 21. Salty groundwater samples (TDS 3000–5000 mg/l) represented 46% of the total groundwater samples (Nos. 3, 4, 6, 7, 10, 11, 13, 14, 15, 23 and 24). The rest of the groundwater samples (8% No. 9 and 17) aquifer are considered saline water (TDS 5000–10,000 mg/l). The lowest TDS values were found in the Eastern of the study area.
Groundwater mineralization processes
Correlation analysis was performed using the SPSS software, (Gopinath et al. 2016) to identify the correlation between the major elements versus TDS that contribute to the mineralization of the groundwater, (Table 6). These correlations indicate that the referenced elements contribute significantly to groundwater salinization in this aquifer. these correlations in the case of the groundwater wells show a strong correlation between the values of Na+ and Cl− with the TDS values (R2 = 0.915 for Na+ and 0.89 for Cl−) and a good correlation between Ca2+ and Mg2+ values with TDS values (R2 = 0.72 and R2 = 0.78 respectively) also no correlation designated in case of HCO3 values with TDS (R2 = 0.43). We created correlations between Ca2+ & SO42−, Mg2+ & SO42−, and Ca2+ & Mg2+ to show the many mechanisms causing this mineralization, (Table 6). In the groundwater wells the positive correlation between Ca2+ and SO42− (R2 = 0.72) shows that the dissolution of gypsum is the major process of groundwater salinization, (Najet et al. 2019). This dissolution is also confirmed with the majority of samples by the low saturation indices concerning these minerals (from −2.22 to −0.125, Table 7) and shows a positive relationship (R2 = 0.68) when correlated with the concentrations of Ca2+ + SO42−meq/l, (Fig. 5). However, for all samples, the Ca2+/SO42− ratios are lower than those resulting from gypsum dissolution. Accordingly, we may deduce the existence of another source of SO42−, which is most likely caused by the dissolution of the epsomite mineral (Kearney 1906). This is also confirmed by the positive correlation between Mg2+ and SO42− in the groundwater (R2 = 0.798) (Table6).
About 88% of the groundwater samples have values of the ratio Na+/(Na+ + Cl−) > 0.5, (Table 8), which indicates sodium sources other than halite as ion exchange or from silicate weathering (Hounslow 1995) or from anthropologic sources.
On the other hand, about 92% of the total groundwater samples have values of Ca2+/(Ca2+ + SO42−) < 0.5, (Table 9). It indicates calcium removal by reverse ion exchange or calcite precipitation, (Hounslow 1995).
((Na+ + K+)—Cl−) versus (Ca2+ + Mg2+)—(HCO32− + SO42−) (meq/l) represents the amount of Na+ gained or lost relative to that provided the halite dissolution, whereas (Ca2+ + Mg2+)—(HCO32− + SO42−) (meq/l) represents the amount of Ca2+ and Mg2+ gained or lost relative to that provided by gypsum, calcite, and dolomite dissolution. If ion exchange is a significant composition controlling process, the relation should be a linear slope (Fisher and Mullican 1997). Figure 6 illustrates this relation, in the groundwater samples; indicating a strong level of ion exchange. During this process, Ca2+ in the solution is exchanged with Na+ and vice versa in the sediments, (Elsheikh et al. 2023).
Ion-exchange index [rCl−–r(Na+ + K+)]/rCl− has either negative or positive values (Table 8). The negative values mean that alkaline earth (Ca2+ and Mg2+) in water replace alkalis (Na+ + K+) on the surface of clay minerals in the aquifer and vice versa in case of positive values, (Mohallel and Gomaa 2017; Mohallel 2020; Elshekh et al. 2023). 88% of the groundwater samples have negative values of cation exchange index and vice versa in the case of the rest of the samples (12%) which have positive values of cation exchange index, the results agree with Ca2+/ (Ca2+ + SO42−) < 0.5, which indicates calcium removal by reverse ion exchange or calcite precipitation.
Saturation indices (SI)
The saturation indices (SI) of some proposed minerals were determined to understand the interaction of rocks with circulating groundwater (Parkhurst and Appelo 2013). The chemical analysis data is used to select these minerals. Table 7 and Fig. 7 show the SI calculated values of the selected minerals. The figure illustrates Gypsum and Anhydrite dissolution with negative SI values, whereas iron minerals (Hematite and Goethite) are supersaturated with positive values. Carbonate minerals (Calcite, Dolomite and Aragonite) are insufficiently soluble in groundwater, with values that fluctuate positively and negatively around zero. This confirms the role of the various geochemical processes which were previously identified by studying the relationships of ions with each other in the enrichment or depletion of groundwater with the elements related to these minerals.
Hypothetical salts assemblages
According to Collin (1923) and (Elsheikh et al. 2023) the combination between the major anions and cations reveals the formation of four groups (I, II, III & IV) of hypothetical salts in the groundwater samples (Table 8). The majority of the groundwater samples (84%) characterized by the assemblages II & III that have two or three sulfate salts (more advanced stage) reflecting the meteoric water affected by leaching and dissolution of terrestrial salts (Elsheikh et al. 2023). About 4% of the groundwater samples characterized by the assemblage I represent an earlier stage of chemical development (sample no. 21), than that of assemblages II & III. Also, assemblage I (Three bicarbonate salts) reflects dilution effect, (Mohallel 2009) by fresh water. The rest of the groundwater samples characterized by the assemblages IV (sample No. 2) and V (samples 3 & 10) characterize groundwater affected by marine deposits (Elsheikh et al. 2023).
Hydrochemical facies and evolution
The primary and secondary sources of dissolved ions in the groundwater system are influenced by human-induced activities and the chemical composition and solubility of minerals found in host rocks, (Mgbenu and Egbueri 2019; Deepali et al. 2020b; Subba Rao 2020). Through the interaction of soil, worn, and fractured materials, infiltrated recharge water enters the aquifer system. This interaction provides a detailed picture of the chemical properties and identifies the sources of dissolved salts in the groundwater, (Subba Rao et al. 2012a, 2017). Plotting the results of the analytical data for the groundwater samples on piper’s tri-linear diagram (1944) shows that (Fig. 8) three groundwater types can be distinguished; Na–Cl type, Ca–Cl type, and mixed Ca–Mg–Cl type, which are predominated in the study area. The majority (84%) of the groundwater samples are located in sub-area 7 {Noncarbonate alkali (Na+ + K+) Cl and (Na+ + K+)2 SO4}, where the groundwater is dominated by alkalis and strong acids (Na–Cl type, Primary salinity). This groundwater has one type of water (II, III, and V assemblages of hypothetical salts, two or three of sulfate salts, and one or two chloride salts). This is due to the leaching and dissolution of terrestrial and marine salts. Few of the groundwater samples (8%, samples No. 1, 2, and the Drain sample, (25)) are located in sub-area 9 where no one cation- anion pair exceeds 50 percent. This groundwater has mixed facies (II and IV assemblages of hypothetical salts) which dominate in a few groundwater samples with subordinate marine and terrestrial facies types, (Ca–Mg–Cl type). Samples No. 16 (irrigation canal) and No. 22 (4%) (Close to the Nile River) are located in sub-area 5 (carbonate hardness or temporary hardness or secondary alkalinity) exceeds 50%, that is chemical properties of the surface water dominated by alkaline earth and weak acids {CaMg (HCO3)2}, these samples have a meteoric origin (II). The rest of the groundwater samples (4%, sample No. 10) are located in sub-area 6 (Noncarbonate hardness or secondary salinity), exceeding 50%, Ca–Cl type. This groundwater is affected by marine deposits, (V assemblage of hypothetical salts).
The primary hydrochemical facies of the numerous operations that govern groundwater chemistry could also be illustrated by plotting the constituents of the analyzed water samples on the Chadha diagram (Chadha 1999). Based on the location of water samples in a certain sub-areas of the diagram (Fig. 9), we will figure out that the origin of the groundwater has undergone a process of evolution, starting from the first stage, where the source of recharge from the irrigation canal and Nile water (Ca–Mg–HCO3 type), passing through the second stage, where the water type is Ca–Mg–Cl resulted from Ion–Exchange processes and arriving at the final stage, where the water type is Na–Cl which resulted from leaching and dissolution of marine and terrestrial salts.
Ion ratios
(rNa+/rCl−)
The values of the hydrochemical coefficient (rNa/rCl) for the majority of the groundwater (92%) are more than one this reflects that sodium is predominately over chloride (Table9) which indicates meteoric water origin. The rest of the groundwater samples (8%) have values of (rNa/rCl) less than one this reflects the leaching and dissolutions of marine sediments. The increasing concentration of Na+ and K+ ions from the hydrochemical coefficients Na+/Cl− or (Na+ + K+)/Cl−, more than 1 in the majority of the samples, and Na+/(Na+ + Cl−) more than 0.5 in the majority (88%) of the groundwater samples, (Hounslow 1995) (Table 9). All these ratios indicate other sources of Na+ ions than halite.
Hussin et al. (2016) and Mukate et al. (2020) used the Na+ vs C1− diagram to explain the contribution of Na+ and C1− to the groundwater system through the dissolution of evaporate deposits. According to Fig. 10, the groundwater sampling points must fall along the equiline of Na+: C1− (1:1) if the evaporate dissolution occurs. In the present study area, 8% of the groundwater sampling points are seen along the 1:1 line (Fig. 10), indicating that halite is the prime source for Na+ and C1− ions in the groundwater regime (Wagh et al. 2019; Karunanidhi et al. 2020). The rest of the groundwater sampling points (80%) and (12%) are scattered towards the Na+ and C1− ions, respectively, which indicate the alternative sources of these ions.
The other sources of Na+& K+ ions can be interpreted as cation exchange and/or silicates weathering sources (Hounslow 1995; Mohallel et al. 2019) from the hydrochemical ratios (Table 9). The silicate weathering as a source of (Na+& K+) can be declared by the relations of Ca2+/Na+ versus HCO3−/Na+ molar ratio (Fig. 11).
All the groundwater samples (Fig. 11) show a decrease of Ca2+/Na+ and HCO3−/Na+. This could be explained as the silicate weathering while samples number 22 (main Drain in the study area) has Ca2+/Na+ and HCO3−/Na+ more than one showing ion exchange process (Rahman et al. 2011).
(rCa2+/rMg2+)
Values of the hydrochemical coefficient rCa/rMg, in the groundwater samples range from 0.54 to 1.04 (Table 9) with an average value 0.73. The excess of magnesium over calcium in the groundwater samples reflect leaching and dissolution of marine deposits.
The Ca2+/Mg2+ ratio in mmol/l of groundwater could reveal that the dissolution of calcite and dolomite Ca2+/Mg2+ = 1 indicated the dissolution of dolomite; the ratio > 2 reflected an effect of silicate dissolution. The ratio between 1 and 2 indicated the dissolution of Dolomite (Maya and Loucks 1995). Figure 12 reveal that in all the groundwater in the study area have Na+/Cl− > 1 and Ca2+/Mg2+ < 1 (in mmol/l), indicate Dissolution of Calcite (Table 10).
(rSO4 2−/rCl−)
This ratio serves as a guide for detecting any excess sulfate in the groundwater (due to CaSO4 dissolution or CaCO3 precipitation) associated with sulfate mineral dissolution. The mean value of the (SO42−/Cl−) ratio for the groundwater samples (Table 9) is 0.97.
The solubility of sulfate mineral components gradually increases in the hydrochemical composition by depth and distance from the recharge to the discharge area (Burdon 1958). Consequently, the percolation of meteoric water and the greater distance of recharge area are the main factors in increasing the sulfate concentration by direct action of leaching processes. The (SO42−/Cl−) ratio of the majority (96%) of the groundwater samples in the study area have values more than that in seawater (0.1), indicates the solution of a local terrestrial source of sulfate such as Gypsum and Anhydrite.
Anthropogenic mineralization processes
Few groundwater samples show that K+/Cl− and K+/Na+ are less than 0.03, (Table 10), indicating no other source of potassium. A high SO42−/Cl− ratio (Table 9) in 40% of samples (more than or near to one) is attributed to the dissolution of some minerals that contain sulfate as sodium sulfate (Glauber's salt), magnesium sulfate (Epsom salt), and calcium sulfate (gypsum) and/or application of gypsum fertilizers i.e. influences of anthropogenic activities. Additionally, high sulfate concentrations could corrode plumbing, especially copper piping.
The main dominant anion in the case of saline water is Cl− ion and also the main dominant anion in the case of fresh water is HCO3− ion when we study the relation between them the Revelle coefficient will be expressive, Revelle coefficient Cl−/(HCO3− + CO32−) in meq/l, (Revelle 1941), (Table 11), showed a relative increase in Cl− relative to CO32− & HCO3− indicating the contamination beside carbonate and silicates weathering (Fisher and Mullican 1997). 91% of all groundwater samples showed the effect of contamination by return irrigation saline water besides leaching and dissolution of halite minerals. Referring to the groundwater contamination limits from those suggested by Kallergis (2000). It suffices to state that values that indicate groundwater contamination could be classified as belonging to the categories ranging between groundwater with good quality, uncontaminated level to dangerous level of contamination in the groundwater samples (Table 11).
Environmental stable isotopes
In this study, 18O and 2H have been used to investigate geochemical processes affecting the groundwater to delineate the main source(s) of groundwater recharge. Recently, stable isotopes have been greatly applied to obtain information on the conditions during recharge and to study groundwater mixing (Clark and Fritz 1997). The ultimate goal of the application of these tracer methods is to quantify the groundwater recharge and hence the sustainability of groundwater supply in the study area.
The global meteoric water line has been plotted using the values of δ18O and δ2H. The values of δ 18O in the groundwater samples range between (1.03 to 3.9 ‰) and (6.1 to 28.2 ‰) for δ 2H (Table 12). All the groundwater samples are enriched and have been plotted close to the Nile River water and the irrigation canal samples except sample No. 23 (Fig. 13). This indicates a considerable recharge that comes from the irrigation canals which come directly from the Nile River through the lifting stations in the study area (Mohammed et al. 2016; Mohallel 2020).
Trace elements
Table 13 shows that all the groundwater samples (Table 4) have average concentrations less than the standard limits guidelines for human drinking according to the World Health Organization for drinking water quality (WHO 2017) and also less than the recommended limits for the short-term for irrigation according to the Australian guidelines for irrigation water quality (AWG 2012).
Conclusion
The current study discussed the significance of using major ion chemistry, Ion ratios, hypothetical salts, groundwater origin, and environmental stable isotope tools to delineate the variables influencing groundwater quality in El Saieda Basin area. The relationship between salinity content and the other ions indicates that sodium, calcium, magnesium, sulfate, and chloride are the effective ions that increase salinity content. Silicate weathering and dissolution of calcite are the main hydrochemical processes affecting the groundwater. Hypothetical salts indicated that the quaternary groundwater aquifer in the study area is highly evolved as it is enriched with terrestrial and marine residuals indicated by the presence of NaCl, MgCl2, MgSO4, CaSO4, Ca(HCO3)2 salts. From the study of the environmental stable isotopes, it is indicated that all the groundwater samples are enriched by 18O and Deuterium which indicates a recharge from the irrigation canals and return flow irrigation water.
References
Abd El-Ghani MM, Huerta-Martínez FM, Hongyan L, Qureshi R (2017) The desert of Egypt. In: Plant responses to hyperarid desert environments. Springer, Cham. https://doi.org/10.1007/978-3-319-59135-3_2
Abdel Kreem WF (2000) Chemical and isotopic investigation of groundwater aquifers in Idfu region on the fringes of Qena Governorate, Dissertation, Faculty of Science, Cairo University
American Society for Testing and Materials, ASTM (2002) Water and environmental technology. Annual book of ASTM standards
Awad AA, Sweed AA (2020) Influence of organic manures on soil characteristics and yield of Jerusalem artichoke. Commun Soil Sci Plant Anal 51(7):1–13
AWG (2012) Australian water guidelines for irrigation, the State of Queensland. Department of Environment and Heritage Protection. https://www.ehp.qld.gov.au/water/guidelines/, 2012–2018
Bach A (2008) (Lecturer) (Class Notes). The soil environment. Western Washington University
Burdon DJ (1958) Metasomatism of groundwater at depth. UNESCO course on hydrogeology, Desert Institute, Cairo, Egypt
Chadha D (1999) A proposed new diagram for geochemical classification of natural waters and interpretation of chemical data. Hydrogeol J 7:431–439
Clark ID, Fritz P (1997) Environmental isotopes in hydrogeology. CRC Press Florida, p 320
Collin AG (1923) Geochemistry of oil field waters. Elsevier, Science Publishing Company, Amesterdam, The Netherlands, p 475
Deepali M, Malpe DB, Subba Rao N, Sunitha B (2020) Geochemical assessment of fluoride enriched groundwater and health implications from a part of Yavtmal District. India Hum Ecol Risk Assess: Int J 26:673–694. https://doi.org/10.1080/10807039.2018.1528862
Elsheikh AE, Mohallel SA, Ezzeldin HA, El Ammawy M, Abdel Lateif RM (2023) Hydrogeological and hydrogeochemical insights on the salinization of the shallow groundwater aquifer in Siwa Oasis—Western Desert–Egypt, Scientific African, Vol. 20. https://doi.org/10.1016/j.sciaf.2023.e01742
Elwan AA (2008) Classification and evaluation of soils in wadi EL-Saieda area, Aswan governorate, Egypt. Dissertation, Department of Agriculture soils Science Zagazig, University Egypt
Farid I, Zouari K, Rigane A, Beji R (2015) Origin of the groundwater salinity and geochemical processes in detrital and carbonate aquifers: case of Chougafiya basin (Central Tunisia). J Hydrol 530:508–532
Fisher RS, Mullican WF (1997) Hydrochemical evolution of sodium-sulfate and sodium-chloride groundwater beneath the northern Chihuahuan Desert, Trans-Pecos, Texas. USA Hydrogeol J 5:4–16
Freeze RA, Cherry JA (1979) Groundwater. Prentice Hall, Englewood Cliffs, NJ. USA
Gattacceca JC, Vallet-Coulomb C, Mayer A, Claude C, Radakovitch O, Conchetto E, Hamelin B (2009) Isotopic and geochemical characterization of salinization in the shallow aquifers of a reclaimed subsiding zone: the southernVenice Lagoon coastland. J Hydrol 378:46–61
Gopinath S, Srinivasamoorthy K, Vasanthavigar M, Saravanan K, Prakash R, Suma CS, Srinivasamoorthy K, Vasanthavigar M, Senthilnathan D (2016) Hydrochemical characteristics and salinity of groundwater in parts of Nagapattinam district of Tamil Nadu and the Union Territory of Puducherry, India. Carbonates Evaporites 33:1–13. https://doi.org/10.1007/s13146-016-0300-y
Hounslow A (1995) Water quality data: analysis and interpretation. Oklahoma State University Oklahoma, U. S. A.J. Environment and Earth Science, p 400
Huneau F, Dakoure D, Celle-Jeanton H, Vitvar T, Ito M, Traore S, Compaore NF, Jirakova H, Le Coustumer P (2011) Flow pattern and residence time of groundwater within the south-eastern Taoudeni sedimentary basin (Burkina Faso, Mali). J Hydrol 409:423–439
Hussin NH, Yusoff I, Tahir W, Mohamed I, Ibrahim AIN, Rambli A (2016) Multivariate statistical analysis for identifying water quality and hydrogeochemical evolution of shallow groundwater in Quaternary deposits in the Lower Kelantan River Basin, Malaysian Peninsula. Environ Earth Sci 75:1081. https://doi.org/10.1007/s12665-016-5705-3
Kallergis G (2000) Applied environmental hydrogeology, 2nd et al. Ed. Technical chamber of Greece: Athens. 2: 345
Karunanidhi D, Aravinthasamy P, Deepali M, Subramani T, Bellows CB, Li P (2020) Groundwater quality evolution based on geochemical modeling and aptness testing for ingestion using entropy water quality and total hazard indexes in an urban-industrial area (Tiruppur) of Southern India. Environ Sci Pollut Res. https://doi.org/10.1007/s11356-020-10724-0
Kearney TH (1906) Date varieties and date culture in Tunis. US Department of Agriculture, Bureau of Plant Industry, Bulletin no. 92, Issue 6
Kehew AE (2001) Applied chemical hydrogeology. Prentice Hall, Upper Saddle River, New Jersey 07458, p 368
Little OH, Attia MI (1943) The development of the Aswan district, with notes on the minerals of south eastern Egypt. Geol. Survey Egypt, Cairo, pp 107
Maya AL, Loucks MD (1995) Solute and isotopic geochemistry and groundwater flow in the central Wasatch Range, Utah. J Hydrol 172:31–59
Mgbenu CN, Egbueri JC (2019) The hydrogeochemical signatures, quality indices and health risk assessment of water resources in Umunya district, Southeast Nigeria. Appl Water Sci 9:1–19. https://doi.org/10.1007/s13201-019-0900-5
Mohallel SA (2020) Hydrogeochemical study for sustainable development in Wadi El-Mathula East Qift city, Eastern Desert, Egypt. Int J Environ 09(02):60–82
Mohallel SA, Gomaa MA (2017) Studying the impacts of groundwater evolution on the environment west Qena city, Egypt. Arab J Geosci 10:372. https://doi.org/10.1007/s12517-017-3151-5
Mohallel SA, Abdella HF, Habibah AZ (2019) Hydrogeochemical assessment of groundwater quality at Wadi Abbadi, southern part of eastern desert, Egypt. Curr Sci Int 8(2019):422–438
Mohallel SA (2009) Hydrochemistry and treatment of groundwater in the area between Mersa Matruh and El Salloum, Egypt. Dissertation, Al Azhar university, Cairo, Egypt
Mohamed A, Sylvain O (2007) Suspended sediment transport in a semiarid watershed, Wadi Abd, Algeria (1973–1995). J Hydrol 343(3–4):187–202
Mohammed A, Krishnamurthy RV, Alan EK, Crossey LJ, Karl KK (2016) Factors affecting the stable isotopes ratios in groundwater impacted by intense agricultural practices: a case study from the Nile valley of Egypt. Sci Total Environ 573:707–715
Mukate SV, Panaskar DB, Wagh VM, Baker SJ (2020) Understanding the influence of industrial and agricultural land uses on groundwater quality in semiarid region of Solapur, India. Environ Dev Sustain 22:3207–3238. https://doi.org/10.1007/s10668-019-00342-3
Najet T, Belgacem A, Adel K (2019) Assessment of groundwater quality of El Ouara aquifer (southeastern Tunisia), geochemical and isotopic approaches. Arab J Geosci 12:116
Parkhurst DL, Appelo C (2013) Description of input and examples for PHREEQC version 3—a computer program for speciation, batchreaction, one-dimensional transport, and inverse geochemical calculations. US Geol Surv Tech Methods 6:497
Piper AM (1944) A graphic procedure in the geochemical interpretation of water analyses. Trans Am Geophys Union 25:914–923
Planation surface (2008) In Encyclopædia Britannica. Retrieved 11 Oct 2008, from Encyclopædia Britannica Online: http://www.britannica.com/ EBchecked/topic/ 462878/ planation-surface
Rahman MA, Majumder RK, Rahman SH, Halim MA (2011) Sources of deep groundwater salinity in the south western zone of Bangladesh. Environ Earth Sci 63:363–373
Revelle R (1941) Criteria for recognition of sea-water in groundwater [M]. Trans Amer Geophys Union (Part III):595–596
Said R (1981) The geological evolution of the River Nile. Springer Verlag
Said R (1962) The geology of Egypt. Elsevier, Amsterdam, New York U.S., p 377. Publishing Co., The Netherlands
Shata AA (2008) Personnel communication and discussion with Prof. Dr. Abdu Shata
Shukri NM, Ayouty MK (1953) The mineralogy of the Nubian sandstone in Aswan. Bull Inst Desert Egypt 3(2):65–88
Subba Rao N (2020) Spatial distribution of quality of groundwater and probabilistic noncarcinogenic risk from a rural dry climatic region of South India. Environ Geochem Health. https://doi.org/10.1007/s10653-020-00621-3
Subba Rao N, Chaudhary M (2019) Hydrogeochemical processes regulating the spatial distribution of groundwater contamination, using pollution index of groundwater (PIG) and hierarchical cluster analysis (HCA): a case study. Groundw Sustain Dev 9:100238. https://doi.org/10.1016/j.gsd.2019.100238
Subba Rao N, Subrahmanyam A, Ravi Kumar S, Srinivasulu N, Babu Rao G, Surya Rao P, Venktram RG (2012) Geochemistry and quality of groundwater of Gummanampadu Sub-basin, Guntur District, Andhra Pradesh, India. Environ Earth Sci 67:1451–1471. https://doi.org/10.1007/s12665-012-1590-6
Subba Rao N, DeepaliM DA, Chandana I, Sunitha B, Ravindra B, Balaji T (2017) Geochemical characteristics and controlling factors of chemical composition of groundwater in a part of Guntur district, Andhra Pradesh, India. Environ Earth Sci 76:747
Sweed AA, Ghallab A, Gameh MA, Naser H (2023) evaluation of soil chemical properties variability using GIS technique in wadi saida area-Aswan-Egypt. J Agric Environ Sci 22(1):309–337
UNDP/UNESCO/NARSS/EGSMA (2002) Joint project for capacity building of the Egyptian geological survey & mining authority and for the sustainable development of the south valley and Sinai, Egy/97/011.
USAID (1978) Geological map of the Aswan quadrangle, Ministry of Industry And Mineral Resources, The Egyptian Geological Survey And Mining Authority, Egypt
Wagh VM, PanaskarDB JA, Mukate SV, Muley AA, Kadam AK (2019) Influence of hydrogeochemical processes on groundwater quality through geostatistical techniques in Kadava River basin. Western India Arab J Geosci 12:7. https://doi.org/10.1007/s12517-018-4136-8
WHO (2017) Guidelines for drinking-water quality. World Health Organization, Geneva
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB). The author received no specific funding for this work.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
There is no any conflict of interest in publish of this Manuscript.
Ethical approval
All procedures performed in this study were in accordance with the ethical standards of the instructional national research committee and its later amendments or comparable ethical.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Mohallel, S.A. Hydrogeochemical tracers to assess the groundwater in El Saieda Basin, western desert, Aswan, Egypt. Appl Water Sci 14, 157 (2024). https://doi.org/10.1007/s13201-024-02147-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13201-024-02147-w