Abstract
The removal of phenolic compounds from the water was of great importance due to their high toxicity. In this study, the separation of phenol from an aqueous environment by pervaporation technique using (PVA) polyvinyl alcohol, (CA) cellulose acetate, and (PVDF) polyvinylidene fluoride membranes was tested. The effect of feed concentration up to 9000 ppm, operating temperature from 25 to 65 °C, and flow rate ranging from 2 to 6 L h−1 on the separation performance was investigated. It was found that the CA membrane possessed a higher water flux of 348.25 kg m−2 h−1 and a separation factor of 49 compared to PVDF, and PVA/SA membranes at 65 °C and a flow rate of 6 L h−1. The properties and morphology of membranes were observed using mechanical properties, contact angle, X-ray diffraction (XRD), Fourier transform infrared spectroscopy (FTIR), and scanning electron microscopy (SEM). The results showed that CA has a lower contact angle of 48.3° indicating the hydrophilicity nature of the membrane, which enhances the separation process and explains the increases of water flux. Moreover, the mechanical properties test indicated that the mechanical strength of CA has a maximum tensile strength of 65.5 MPa and an % elongation of 48% compared to PVDF and PVA/SA which indicates lower roughness, manifesting its improved anti-fouling properties.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
One of the most common sources of pollution is phenol-contaminated effluents generated during the manufacture of various chemical compounds and refinery industries. The elimination of phenol is of major importance in wastewater treatment because phenolics, even at low concentrations, have the potential to damage people, wildlife, and the environment. The Environmental Protection Agency's (EPA) water purity standards include a regulation level of one part per billion (ppb) for phenol content. The toxicity tolerance for both humans and marine species has been observed to be between 9 and 25 mg L−1 (Wibawa Hendra Saputera 2021).
Wastewater separation techniques are critical to producing high-quality products with acceptable yields at a cheap cost. Conventional methods of separating mixtures, such as distillation, extraction, or absorption, as well as crystallization processes and combinations of these methods, are well known; however, many of them are costly energy, so alternative methods, such as membrane-based separations, are being researched. Membrane methods are reliable and cost-effective for separation. It consumes less energy than other methods such as distillation because mixture separation may be done at considerably lower temperatures than the boiling temperatures of the components (Zeitoun 2020). The development of appropriate membrane materials and modules is important for effective competition with current separation technologies. The most important membrane technologies used to remove phenols from wastewater are extractive membrane bioreactors and hollow fiber membranes; photocatalytic membrane reactors; high-pressure membrane processes such as nanofiltration, reverse osmosis, and pervaporation; and membrane distillation (Loh et al. 2016).
In this work, a single-step pervaporation technique is integrated with liquid–liquid phase separation. Pervaporation is recognized as an efficient procedure for separating azeotropic mixtures, chemicals with low boiling points, and mixes containing heat-sensitive compounds. In recent years, various studies have been conducted using polymeric membranes (Mohammadi et al. 2015). A good pervaporation membrane material will have a high permeation flux and separation factor. The success of the pervaporation process is dependent on membranes that have high permeability, selectivity, and mechanical strength (Satyanarayana 2012). Table 1 lists current investigations on the recovery of phenol and organic chemicals from wastewater streams using the pervaporation method.
There is little information about the removal of phenol using cellulose acetate till now. Most of the studies using cellulose acetate incorporated different polymeric materials to enhance the separation efficiency. The present research aims to study the effect of phenol separation using CA and compare it with different polymeric membranes and study the effect of surface nature; hydrophilicity/hydrophobicity on the phenol separation. The experimental work explores the effect of varying phenol concentrations in the feed mixture on membrane flux, selectivity, permeate, and separation factors.
Materials and method
Materials
The material specifications and suppliers are given in (Table 2).
Membrane preparation
PVA/SA membrane
The 2.5 wt% PVA solutions and 2wt% SA were prepared by dissolving separately 2.5 g PVA and 2 g SA in 100 ml deionized water. then 50:50 proportion of the two solutions were mixed with continuous stirring for 2 hr. to ensure the complete mixing of the two polymers followed by drying the polymers at room temperature. For the crosslinking, the prepared PVA/SA was immersed in a solution containing 10% GA (as a crosslinking agent) and 0.05 vol% HCl in acetone (as a catalyst). The prepared PVA/SA membrane was immersed in methanol for 24 hr. to remove any unreacted GA, rinsed with water, and then dried at room temperature (Dave and Nath 2017a).
Cellulose acetate membrane
The manufacturing of the cellulose acetate membrane involved the implementation of the phase inversion method for the CA membrane. A solvent blend containing equal parts of acetone and dimethylformamide was employed to dissolve a 25% weight fraction of cellulose acetate (CA). The mixture was stirred periodically for a duration of eight hours. Following a 24-hour waiting period, any air bubbles that could have been retained in the polymeric solution were effectively removed. The polyester support functioned as the operative substrate during the casting procedure. Subsequently, the process of coagulation occurs within a cold-water bath maintained at a temperature of 5 °C. Subsequently, the fabricated membrane underwent annealing in a water bath maintained at a temperature of 85 ºC for a duration of five minutes (Algieri and Chakraborty 2022).
Method
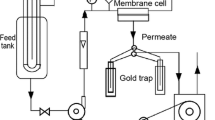
The pervaporation apparatus consisted of a permeation cell made of stainless steel, a constant temperature bath, and glass tubes for condensing and collecting the permeate vapor. The membrane area in contact with liquid was about 16 cm2. The downstream pressure was maintained from 200 mbar. Pervaporation experiments were carried out at a feed temperature from 25 to 55 °C, and feed concentration ranged from 1000 to 9000 ppm. The experiments were carried out on a Pervaporation apparatus as shown in Fig. 1. The heated feed mixture at the desired temperature was continuously circulated over the membrane using a circulating peristaltic pump from the feed tank, while a vacuum is applied on the downstream side of the membrane. The liquid permeant vaporizes between the upstream and downstream sides of the membrane where it is obtained as vapor and condensed (Ong et al. 2016). The permeate was collected and analyzed using an Agilent UV–VIS spectrophotometer. The analysis of permeate was carried out according to ASTM 5530C (Cristian perla Martinez 2010). The separation factor (α) and flux (J) were determined using the following equations
where X and Y are the weight ratio of each component respectively in feed and permeate.
where W is the wt. of the permeate (kg), A is the effective membrane area (m2).
Characterization
The investigation of the three membranes' properties was discussed using a combination of techniques, including scanning electron microscopy (SEM; S4800, Hitachi, Japan) at an accelerating voltage of 20 kV, contact angle, Fourier-transform infrared spectroscope (FTIR; Tensor 37, Bruker, Germany) with a wave number range of 500–4000 cm−1, a scan number of 32, and a resolution of 4 cm−1, and X-ray diffractometer (D8 ADVANCE, Bruker, Germany) using Cu-Ka radiation (k¼1.5438 A°).
Result and discussion
Fourier-transform infrared spectroscopy (FTIR)
It was used to characterize the functional groups of the three developed membranes. Figure 2a–c show the visible characteristic peaks of PVA, PVDF, and CA membranes, respectively. The peaks of the PVA/SA membrane are indicated in Fig. 2a at 3422.23, 1639.45, and 1100.43 cm−1. The peak at 3422.23 cm−1 represents the peak of intramolecular hydrogen bonding (O–H) (Wang et al. 2021). FTIR spectra of PVDF thin films are also shown in Fig. 2b. Peaks at 435, 502, 631, 724, 792, 1120, 1384, and 1446 cm−1 are used for α -phase identification, while peaks at 874 cm−1 are β-phase fingerprints. α–phase molecular vibration peaks are very close to β -phase. As a result, separating two phases by using FTIR spectroscopy is extremely difficult (Zeitoun 2020).
The spectrum of FTIR for the CA membrane is shown in Fig. 2c, with a peak at 3420 cm−1 representing the carboxylic acid group. The vibration of the C=O group was assigned a strong peak around 2112 cm−1, and the stretching mode of the C=C was assigned at 1640 cm−1. The distinctive peak at around 724 cm-1 reflected the bending of the C–H group (Zafar and Ali 2012b).
X-ray diffraction analysis (XRD)
The X-ray of PVA/SA, PVDF, and CA membranes are illustrated in Fig. 3a–c. In Fig. 3a, the XRD of the PVA/SA nanofiber membranes showed three peaks at 17.54°, 22.6°, and 25.94°. Crosslinks typically reduce the chain mobility of a polymer as the crosslinking reaction progresses. As a result, PVA showed a sharp peak with high intensity at 2 θ = 17.54°, and the resulting semi-crystal peak (SA) was found at 2 θ = 22.6 (Hezma et al. 2023). Because of the presence of an amorphous structure, the wide range from 17.54° to 25.94° can provide information on the presence of a component. The X-ray diffraction pattern of PVDF thin films is shown in Fig. 3b. The peaks in the XRD pattern at 2 θ = 17.9 and 26.26 confirm the presence of -phase (Satapathy et al. 2011).
Furthermore, the XRD of the CA nanofiber is described in Fig. 3c, which shows two peaks with nearly identical intensities at 2 θ = 23.34 and 26.6, which are also typical cellulose peaks (Boccaccio 2002).
Scanning electron microscopy (SEM)
Figure 4a, b illustrates the cross-sectional surface structure of the cellulose acetate (CA) membrane, indicating its composition of two layers exhibiting different pore sizes. The membrane that is synthesized exhibits anisotropic characteristics, wherein the outer layer (1) consists of smaller pores, while the inner layer (2) consists of slightly bigger pores. In comparison to the total thickness found in the inner supporting layer, the skin's outermost layer exhibits a relatively reduced thickness. Having a dense arrangement of pores near the skin, reinforced by a spongy microporous structure, is considered advantageous due to its capacity to enhance membrane permeability (Hoshi et al. 1997).
The surface structure of the crosslinked PVA/SA membranes was depicted in Fig. 4 (c-d), revealing a heterogeneous arrangement of the nanofibers characterized by a smooth surface and the presence of many spaces between the fibers. The formation of a dense network structure with comparatively smaller pore size was attributed to the increased involvement of molecular chains in the crosslinking reaction (Chung et al. 1998).
Figure 4e–f demonstrates the PVDF membrane's properties. The PVDF membrane has a smaller mean pore size, resulting in a significantly greater liquid entry pressure and excellent anti-wetting properties. In addition to its high porosity, the PVDF membrane has a dense and smooth top surface, as shown in Fig. 4f (Liu et al. 2018).
Contact angle
The hydrophilicity of membranes was determined using a contact angle test. (Table 3) shows that the test was performed on three different membrane samples: PVA/SA, PVDF, and CA. The PVDF membrane's water contact angle was 74.8°, indicating low wettability on the surface (highest contact angle value) since hydrophobicity increases with contact angle (Vetrivel et al. 2021). Furthermore, the hydrophilicity of SA increased when 2.5% PVA was added, and the contact angle decreased to 64.8°, increasing PVA/SA permeability (Mallakpour and Behranvand 2017). Furthermore, the figure shows that CA has a lower contact angle of 48.3°. This indicates that the CA membrane is more hydrophilic than the other membranes.
Mechanical properties
The mechanical properties of the CA, PVA/SA, and PVDF membranes are illustrated in Fig. 5. The maximum tensile strength at break followed the same pattern as the elongation at break of three membranes. CA has a maximum tensile strength of 65.5 MPa and a % elongation of 48% compared to PVDF and PVA/SA tensile strengths of 55.1, 47.8 MPa, and % elongation of 36.3, and 35.2%, respectively. This could be because CA and PVDF have a more porous membrane structure than PVA/SA, which has numerous voids between the fibers, reducing its stability (Zeitoun et al. 2022).
The impact of feed temperature on various membranes
Figures 6, 7, 8 illustrates the impact of differing feeding temperatures on the separation factor of phenol, as well as the water and phenol fluxes, utilizing membranes made of PVA/SA, PVDF, and CA. The range of temperature from 25 to 65 °C, while the phenol concentration remains constant at 1000 ppm. At an elevated temperature of 65 °C, the separation factor of the CA membrane exhibits a significant rise, rising from 2.1 to 6 In the interim, the separation factor of the PVA/SA and PVDF membranes exhibits a little increase and remains rather constant. Similar results were obtained in the water flux curve illustrated in The water flux of the CA membrane raised from 125 to 295 kg m−2 h−1 with increasing temperature, whereas the water flux of the PVA/SA and PVDF membranes decreased.
These findings are consistent with other studies that show that increasing the temperature makes permeating molecules stronger, thereby increasing permeation flux and the ease of swelling in the membrane structure (Dave and Nath 2017b). The hydrophilic nature of the membranes provides a strong driving force for water affinity, the CA membrane facilitates permeate transport at higher temperatures and increases water flux. As a result of increasing the vapor pressure at higher temperatures, water is preferably dissolved and transported in hydrophilic membranes, increasing water flux.
Meanwhile, Fig. 8 demonstrated that the PVDF membrane has a higher phenol flux of 1 kg m−2 h−1 at 25 °C than CA and PVA//SA because PVDF is a hydrophobic membrane, and thus phenol flux decreases with increasing temperature to 0.79 kg m−2 h−1 at 35 °C and nearly remains constant until 65 °C due to the partial pressure difference created by the temperature gradient in the membrane allows only water to pass through it. This was attributed to an increase in water vapor pressure as the temperature gradient across the membrane increased (Yadav and Yadav 2021). Similar results were obtained in the PVA/SA membrane, SA has a higher tendency to absorb water and causes significant swelling, which affects the PVA pores and slightly increases the flux rate. The increase in the feed temperature affects the thermal mobility of the polymeric chain, increasing the diffusion rate of the permeating molecules (Rhimi et al. 2021).
The impact of feed concentration on various membranes
Figures 9, 10, 11 illustrate the effect of feed concentration on the separation factor of the three membranes. The operating conditions were at 65 °C and the feed concentrations ranged from 1000 to 9000 ppm. Figures 9, 10 showed that the separation factor and water flux of the CA membrane increased from 5.2 to 38.2 and 233.5 to 274.7 kg m−2 h−1 respectively with increasing phenol concentration. Pervaporation is a solution-diffusion process, but it appears clear that the membrane's high selectivity is primarily due to its hydrophilicity nature. This is consistent with the general observation that when organic compounds are separated from dilute aqueous solutions by pervaporation using organophilic membranes, their preferential sorption in the membrane often results in selective permeation (Hao et al. 2009).
In dilute solutions, it is observed that the higher phenol concentration in the feed, increases the driving force and the permeability of the phenol membrane as well as decreases for water permeation, but the decrease is relatively low because phenol is in less concentration. As a result of pervaporation theory and the selectivity of CA, a hydrophilic membrane, the transport of molecules is controlled not only by the sorption step but also by diffusion through the membrane. Water molecules are smaller than phenol molecules and are expected to diffuse more freely through the membrane (Zafar and Ali 2012a).
Figures 10, 11 show separation factor water flux of PVA/SA increased from 0.15 to 10.2 and 81.1 to 128.1 kg m−2 h−1 but at a lower rate compared to the CA membrane. This can be explained due to the higher selectivity of the SA to water which causes the membrane to swell during the pervaporation process and decreases the membrane mobility and diffusivity of phenol permeating through it and the flux will be less increased. However, Fig. 11 demonstrated that the highest phenol flux was for the PVDF membrane due to the PVDF membrane's higher selectivity to phenol (Vatani et al. 2018).
Effect of flow rate on different membrane
Figures 12, 13, 14 explain the behavior of increasing flow rate of aqueous solution through the CA, PVDF, and PVA/SA membranes at a phenol concentration of 9000 ppm and temperature of 65 °C, and flow rate ranged from 2 to 6 L h−1. Figure 12 shows that the separation factor of the CA membrane increases with flow rate due to the velocity increases therefore Reynold‘s number and the rate of water transfer from the bulk solution to the membrane surface increased and subsequently, the water flux and separation factor enhanced (Khatinzadeh et al. 2016).
As observed from Figs. 12 and 13, by increasing the flow rate of feed from 2 to 6 L h−1, the PVA/SA membrane selectivity and separation factor slightly increased and almost constant. Increasing flow rate leads to the increased penetration of water molecules However, as the results show, the variations in flow rate in the PVA/SA membrane are less pronounced because the swelling effect of SA inhibits the penetration and mobility of water molecules, thereby affecting the flow rate (Alanezi et al. 2016).
The impact of the feed flow rate on the phenol flux for PVDF was examined in Fig. 14. As the feed flow rate increases, so does the PVDF membrane's phenol flux. This rise is the result of a higher Reynolds number, which increases turbulence and the flow's greater mixing in the channels. This suggests that more turbulent flow increases the driving force for evaporation by decreasing the thickness of the boundary layers for both temperature and concentration (i.e., the boundary layer resistance). Turbulence further speeds the phenol from the bulk feed to the membrane surface by raising the convective mass transfer coefficient at the feed boundary layer, which is directly related to Reynold number (Xu et al. 2016).
Membrane performance
The performance of the CA, PVA/SA, and PVDF membranes for the removal of phenol from industrial wastewater at a concentration of 9000 ppm and flow rate of 6 L h−1 at 65 °C is investigated in Fig. 15 and (Table 4). The CA was found to have a higher separation factor of 52 due to its hydrophilic properties with spongy micropores, which increase the separation factor and increase water flux in permeate. Due to its hydrophobicity and phenol selectivity, the PVDF membrane has a higher phenol flux than the CA membrane, reaching 3.3 kg m−2 h−1.
Conclusion
The present study investigated the removal of phenol from an aqueous environment utilizing membranes made of PVA/SA, PVDF, and CA through the pervaporation process.
-
The three membranes were characterized using scanning electron microscopy (SEM), X-ray diffraction Analysis (XRD), Fourier transforms infrared spectroscopy (FTIR), and contact angles which indicate the hydrophilicity structure of membranes. Also, the optimum fluxes and separation factor measurements were conducted using synthetic solutions under certain operating conditions, including a phenol concentration of 9000 ppm, temperature of 65 °C, and a flow rate of 6 L h−1.
-
SEM results showed that the CA membrane has a spongy structure which enhances permeability compared to PVA/SA and PVDF membranes which have dense structures.
-
The CA membrane has a maximum tensile strength compared to PVA/SA and PVDF due to its high pore size.
-
The CA membrane possessed a higher water flux of 348.25 kg m−2 h−1 and a separation factor of 49 compared to PVDF, and PVA/SA membranes at 65 °C and a flow rate of 6 L h−1. This can be attributed to the higher porosity and hydrophilicity structure of the CA membrane. Meanwhile, the PVDF membrane indicates a higher phenol flux of 3.3 kg m−2 h−1 compared to the other membranes due to the hydrophobicity structure of the membrane.
-
CA membrane is highly recommended for phenol removal industrial wastewater than PVA/SA and PVDF membrane.
Future work
-
Use industrial wastewater for this application.
-
Examine the membrane fouling test and evaluate how vacuum pressure affects permeation and separation.
-
Compare the performance of cellulose acetate membranes made from organic materials like sugarcane bagasse.
References
Alanezi AA, Abdallah H, El-Zanati E, Ahmad A, Sharif AO (2016) Performance investigation of o-ring vacuum membrane distillation module for water desalination. Corp Jf Chem. https://doi.org/10.1155/2016/9378460
Asiri AM, Petrosino F, Pugliese V, Khan SB, Alamry KA, Alfifi SY, Marwani HM, Alotaibi MM, Algieri C, Chakraborty S (2022) Synthesis and characterization of blended cellulose acetate membranes. Polymers 14:4
Boccaccio T, Bottino CG, Piaggio PJ (2002) Characterization of PVDF membranes by vibrational spectroscopy. Membr Sci 210(2):315–329
Chung TS, Loh K-C, Goh SK (1998) Development of cellulose acetate membranes for bacteria immobilization to remove phenol. J Appl Polym Sci 68:1677–1688
Dave HK and Nath K (2017) Effect of temperature on eater- acetic acid binary mixture pervaporation. J Sci Ind Res 217–222
Dave HK, Nath K (2017) Effect of temperature on pervaporation dehydration of water-acetic acid binary mixture. J Sci Ind Res 76:217–222
Gupta V, Raja C, Anandkumar J (2020) Phenol removal by novel choline chloride blended cellulose acetate-fly ash composite membrane. Periodica Polytech, Chem Eng 64(1):116–123
Hao X, Pritzker M, Feng X (2009) Use of pervaporation for the separation of phenol from dilute aqueous solutions. J Membr Sci. https://doi.org/10.1016/j.memsci.2009.02.036
Hezma AM, Shaltout WA, Kabary HA, Gamal SE-B, Abdelrazzak AB (2023) Fabrication, characterization and adsorption investigation of nano Zinc Oxide-Sodium Alginate beads for effective removal of Chromium (VI) from aqueous solution. J Inorg Organometal Polym Mater 33:400–1408
Hoshi M, Kogure M, Saitoh T, Nakagawa T (1997) Separation of aqueous phenol through polyurethane membranes by pervaporation. J Appl Polym Sci 65:469–479
Kansea NG, Dawandea SD (2017) Separation of ethanol/water (Azeotropic mixture) by pervaporation using PVA membrane. Mater Today: Proc 4:10520–10523
Khatinzadeh G, Mahdyarfar M, Mehdizadeh A, Esmailzadeh A, Sattari A (2016) Effects of permeate pressure and feed flow rate on benzene dehydration by pervaporation. J Pet Sci Technol 6(2):30–36
Li D, Yao J, Sun H, Liu B, Li D, van Agtmaal S, Feng C (2018) Removal of phenolic compounds from industrial waste water based on membrane-based technologies. Chem Eng Res Des 132:424
Liu Y, Xiao T, Bao C, Zhang J, and Yang X (2018) Performance and fouling study of asymmetric PVDF membrane applied in the concentration of organic fertilizer by direct contact membrane distillation (DCMD), Membranes
Loh CH, Zhang Y, Goh S, Wang R, Fane AG (2016) Composite hollow fiber membranes with different poly (dimethylsiloxane) intrusions into substrate for phenol removal via extractive membrane bioreactor. J Membr Sci 500:236–244
Martinez CP (2010) Analysis of phenols in water samples using chloroform extraction and colorimetric detection, Approved by standard method committee
Mallakpour S, Behranvand V (2017) Chapter 10 – Green Hybrid Nanocomposites from Metal Oxides, Poly (Vinyl Alcohol) and Poly (Vinyl Pyrrolidone): Structure and Chemistry, Woodhead Publishing, pp 263–289
Meikhail MS, Hezma AM, Mohamed BA (2018) Characterization and physical studies of Poly (vinyl alcohol)/Sodium alginate/ multiwall carbon nanotube biocomposites. J Text Color Polym Sci 15(1):95–108
Mohammadi S, Kargari A, Sanaeepur H, Abbassian K, Najafi A, Mofarrah E (2015) Phenol removal from industrial wastewaters: a short review. Desaline Water Treat 53:2215–2234
Ong YK, Shi GM, Le NL, Tang YP, Zuo J, Nunes SP, Chung T-S (2016) Recent membrane development for pervaporation processes. Prog Polym Sci. https://doi.org/10.1016/j.progpolymsci.2016.02.003
Rhimi A, Zlaoui K, Van der Bruggen B, Horchani-Naifer K, Ennigrou DJ (2021) Synthesis and characterization of crosslinked membranes based on sodium alginate/polyvinyl alcohol/graphene oxide for ultrafiltration applications. Desalin Water Treat 230:204–218
Saputera WH, Putrie AS, Esmailpour AA, Sasongko D, Suendo V, Mukti RR (2021) Technology advances in phenol removals: current progress and future perspectives. Catalyst 11(8):998
Satapathy S, Santosh Pawar PK, Gupta KBR, Varma, (2011) Effect of annealing on phase transition in poly (vinylidene fluoride) films prepared using polar solvent. Bull Mater Sci 34(4):727–733
Satyanarayana SS (2012) Phosphorylated chitosan membranes for the separation of ethanol-water mixtures by pervaporation. Carbohydr Polym 87:1569–1574
Vatani M, Raisi A, Pazuki G (2018) Pervaporation separation of ethyl acetate from solutions poly(ether-block-amide) / polyethersulfone membrane. R Soc Chem RSC Adv 8:4713–4725. https://doi.org/10.1039/C7RA13382K
Vetrivel S, Saraswathi MSSA, Rana D, Divya K, Nagendran A (2021) Cellulose acetate ultrafiltration membranes customized with copper oxide nanoparticles for efficient separation with antifouling behavior. J Appl Polym Sci. https://doi.org/10.1002/app.49867
Wang C, Zuo Q, Wang L, Long B, Salleh KM, Anuar NIS, Zakaria S (2021) Diameter optimization of polyvinyl alcohol/sodium alginate fiber membranes using response surface methodology. Mater Chem Phys 271:124969
Xu W, Shen R, Yan Y, Gao J (2016) Preparation and characterization of electrospun alginate/PLA nanofibers as tissue engineering material by emulsion eletrospinning. J Mech Behav Biomed Mater 65:428–438
Yadav A, Yadav P, Labhasetwar PK, Shahi VK (2021) CNT functionalized ZIF-8 impregnated poly (Vinylidene Fluoride-Co-Hexafluoropropylene) mixed matrix membranes for antibiotics removal from pharmaceutical industry wastewater by vacuum membrane distillation. J Environ Chem Eng 9(6):106560
Yanhui Wu, Tian G, Tan H, Xiaoting Fu (2013) Pervaporation of phenol wastewater with PVDF–PU blend membrane. Desalin Water Treat 51(25–27):5311–5318
Zafar M, Ali M, Khan SM, Jamil T, Butt MTZ (2012a) Effect of additives on the properties and performance of cellulose acetate derivative membranes in the separation of isopropanol/water mixtures. Desalination 285:359–365
Zafar M, Ali M, Khan SM, Jamil T, Butt MTZ (2012b) Effect of additives on the properties and performance of cellulose acetate derivative. Desalination 285:359–365
Zeitoun Z, El-Shazly AH, Nosier S, Elmarghany MR, Salem MS, Taha MM (2020) Performance evaluation and kinetic analysis of photocatalytic membrane reactor in wastewater treatment. Membranes 10:276
Zeitoun Z, El-Shazly AH, Nosier S, Elmarghany MR, Salem MS, Taha MM (2022) Electrospinning of polyvinylidene fluoride membranes: effect of membrane composition and fabrication conditions. Egypt J Chem 65(2):41–50
Acknowledgements
The authors gratefully acknowledge the Alexandria University- National Research Centre (NRC) cooperation program team for their significant support that helped me to finish this research
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Ethical approval
This article does not contain any studies involving human participants performed by any of the authors.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Eldemerdash, U., Dandash, A., Nosier, S. et al. Investigation of different polymeric membranes for removal of phenol from aqueous environment using pervaporation technique. Appl Water Sci 14, 70 (2024). https://doi.org/10.1007/s13201-024-02136-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13201-024-02136-z