Abstract
Management and protection of regional water resources requires an understanding of the hydrological characteristics and water quality changes. In this study, we combined isotopic, geochemical and hydrometric measurements to investigate hydrological characteristics and water quality changes during the interactions between surface water and groundwater in mountain river valley. Our results showed that the stable isotope values in most of the groundwater and river water samples were located above the middle of the local meteoric water line in a mountain river valley. The hydrochemical types of most of the groundwater and river water samples were Ca–Mg–HCO3 and were primarily dominated by rock weathering. The hydrochemical compositions of groundwater and river water were mainly affected by carbonate dissolution and cation exchange, but influences of saltwater intrusion and human activity were found at the lakeside. Precipitation was the main factor affecting the changes in hydrological processes at these groundwater and river water sites and they were also affected by meltwater, soil water and the interaction between groundwater and surface water. The water level of the river increased, and the recharge of the groundwater by river water increased in river valley during the rainy season, which led to an increase runoff path in groundwater. The interaction between the river water and groundwater was affected by the rainfall frequency and intensity, the recharge time of the soil water and the pressure of the river on both sides of the river channels. Additionally, our results indicated that the flow of groundwater into the river will lead to water quality deterioration. The increasing pressure of the river on the groundwater will cause the deterioration of groundwater quality, which will also be affected by saltwater intrusion and human activity. Our results detailed the hydrological characteristics, water quality changes and main influencing factors of the interaction between surface water and groundwater of river valley in mountain, which will be beneficial to promote the reasonable protection of water resources under climate change in the future.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Water is the most precious resource, along with land and energy, for human survival and socioeconomic development (Howells et al. 2013; Mas-Pla and Menció 2019; Ma et al. 2020; Ondrasek et al. 2021; Wada et al. 2014). However, water shortages are an emergent issue worldwide for several reasons (e.g., climate change, population growth, irrigated croplands, economic development and population) (Florke et al. 2013; Foster and MacDonald 2014; Haddeland et al. 2014; Posthuma et al. 2019; Vorosmarty et al. 2000). Today, more than 1.2 billion people live under water shortages (Howells et al. 2013), and two-thirds of the world’s population will live under water stress by 2025 (UN 2015). Therefore, the global shortage of water poses a serious challenge to sustainable development, but the efficient management of water may alleviate this emergency.
The United Nations reports that more than 1.7 billion people live in river basins (UN 2015) because river water provides freshwater, and freshwater is critical for human production and life. However, the utilization of river water is limited in semiarid and arid regions (e.g., India, Pakistan, northwestern China, the Middle East and North Africa) because of lower precipitation (Wade et al. 2014). In these regions, groundwater is extracted for sustainable food production and economic development. However, the storage and quality of groundwater are rapidly deteriorating due to overexploitation and population, which has caused widespread international concern (Aeschbach-Hertig and Glesson 2012; Feike et al. 2017; Jia et al. 2019; Mariangela et al. 2019; Zaidi et al. 2015). Reasonable exploitation and utilization of surface water and groundwater resources may be the key to promoting sustainable development of water. Therefore, understanding the transport processes and the effects of the interaction between surface water and groundwater on the water environment is crucial for the comprehensive utilization of surface water and groundwater.
Mountains are core components of the global water supply (Somers and Mckenzie 2020). The substantial capacity of groundwater storage and discharge is vital to buffer the streamflow during dry periods in mountain watersheds (Liu et al. 2004; Soulsby et al. 2000; Uhlenbrook et al. 2002). As the global climate changes, temperature changes will dramatically alter mountain hydrological conditions through permafrost degradation, increasing evapotranspiration and reducing water storage in glaciers (Barnett et al. 2005; Immerzeel et al. 2010). Permafrost degradation will deepen the underground runoff path and increase the amount of permafrost meltwater infiltration (Frampton et al. 2013; Ge et al. 2011; Wellman et al. 2013). Focusing on the interaction between groundwater and surface water is beneficial for predicting the change in water under climate change in mountainous areas and formulating timely and effective water resource utilization measures.
The stable isotope ratio (δ18O/ δ16O and δ2H/δ1H) and hydrochemical ions (Ca2+, Mg2+, Na+, K+, Cl−, SO42−, CO32−, HCO3− and NO3−) of water allow the tracing of recharge sources, evolution and flow paths of groundwater and the interaction of surface water and groundwater (Li et al. 2021; Martinez et al. 2015; Rodgers et al. 2005). The integrated application of isotopes and hydrochemical ions is an effective method to reveal hydrological processes, especially the interaction between surface water and groundwater (Cui and Li 2014; Gibson et al. 2005; Raghavendra and Deka 2015). Dogramaci et al. (2012) determined the processes that control surface water and groundwater recharge and quantified the sources of major recharge. Yuan et al. (2020) found that the interaction between rivers and groundwater was completely changed by water transfer by using stable isotopes and hydrochemistry. Zhang et al. (2021) investigated the transport processes between surface water and groundwater in selected tributaries of the Wei River (China).
In this study, stable isotopes and hydrochemical ions from 273 samples were tested and analysed from March 2019 to January 2020 to (1) investigate the spatial and temporal isotopic, geochemical and hydrometric characteristics of precipitation, river water, groundwater and lake water in a river valley, (2) reveal the hydrological characteristics, water quality changes and main influencing factors during the interaction between surface water and groundwater.
Study area
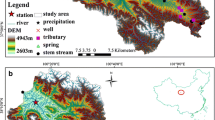
The Qinghai Lake Basin (36°15′–38°20′N, 97°50′–101°20′E) is the largest inland saline lake watershed on the northeastern Tibet Plateau in China, with an area of 29,661 km2 (Fig. 1). The lake is surrounded by the Datong Mountains, Qilian Mountains, Riyue Mountains and Qinghai Nan Mountains (Dong et al. 2019; Fan et al. 2021). The Qinghai Lake Basin lies in a critical transitional zone (Li et al. 2018a, b) that is affected by the East Asian Monsoon in summer (June–August) and the Westerly Circulation in winter (September-May) (Cui et al. 2021; Dong et al. 2019). The three largest rivers in the Qinghai Lake Basin are the Buha River, Shaliu River, and Haergai River, which account for 75% of the surface water inflow into Qinghai Lake.
The Buha River is the largest of these rivers, located in the western Qinghai Lake Basin (Fig. 1). The annual runoff is 7.84 × 106 m3 and the average annual flow is 24.85 m3 s−1. The flood period is from June to September (the flood peaks in July), and the dry period is from December to March. The maximum and minimum flows are 81.33 m3 s−1 and 2.38 m3 s−1, respectively. The width and altitude of the Buha Valley plain are 6–10 km and 3200–3400 m above sea level (m a.s.l.), respectively. The Buha River Valley is wide and flat, and the river terraces are mainly composed of sandy gravel stratum, boulder-clay, clay, sandy conglomerate and granite (Fig. 1).
Sampling and analytical methods
Precipitation samples were collected by using samplers during every precipitation event from March 2019 to February 2020 at the Gangcha meteorological station (3301.5 m a.s.l.) (Fig. 1). Samplers were placed in an open area over 1.5 m aboveground. In total, 142 precipitation samples were collected. Groundwater and surface water (river and lake water) samples were collected once a month from 12 sites from March 2019 to January 2020 (Fig. 1). A total of 131 samples of groundwater and surface water were collected (77 groundwater samples, 43 river water samples, and 11 lake water samples). Groundwater samples were collected after the water conductivity stabilized after 3–5 min of well pumping. River water samples were collected 20 cm below the flowing water surface, and lake water samples were collected 50 cm below the water surface. All samples were filtered using 0.45 μm filters and stored in 50 ml bottles for isotopic analyses. In addition, water samples were stored in 100 ml acidified and not-acidified bottles for cation and anion analyses, respectively. All samples were sealed with sealing film (Parafilm 996, America) and stored in refrigerators (4 ℃).
Stable isotopes were measured using a Los Gatos Research (IWA-45-EP) isotopic water analyser. Cations (K+, Na+, Ca2+ and Mg2+) were measured using an inductively coupled plasma emission spectrometer (Optima -8000). Anions (Cl−, SO42− and NO3−) were measured using ion chromatography (ICS -2000). CO32− and HCO3− were measured in situ using direct titration with phenolphthalein (5 gL−1), methyl orange (1 gL−1), and sulfuric acid (0.01 molL−1). The locations of the sampling sites were acquired using a global positioning system (GPS). The electrical conductivity, total dissolved solids (TDS), temperature and pH of the groundwater were measured in situ by a handheld metre. The water level and temperature of the groundwater were acquired using a water level logger-titanium (ONSET HOBO). Water level and runoff data for the Buha River were downloaded from the Qinghai hydrological information website (http://www.qhsw.org.cn/qhsq.php). Daily precipitation data from the Gangcha weather station were downloaded from the China Meteorological Data Service Centre (http://data.cma.cn/).
The hydrochemical characteristics and evolution of groundwater were revealed using a Piper diagram and the boomerang envelope model (Gibbs 1970; Li et al. 2021; Piper 1944). Piper (1944) drew two triangles on behalf of the cations and anions in order to reflect the main water composition. The apexes of the cation plot are Ca2+, Mg2+ and Na+ + K+. The apexes of the anion plot are SO42−, Cl− and CO32− + HCO3−. A hydrogeochemical categorization of the groundwater, river water, and lake water was carried out based on the major cations and anions in the Piper diagram (Piper 1944). Gibbs (1970) proposed that factors controlling the water composition could be divided into three end-members: rock weathering, atmospheric precipitation, and evaporation/crystallization, based on the chemical composition of surface water samples from across the globe. Landwehr and Coplen (2004) proposed line-conditioned excess (lc-excess = δ2H–aδ18O–b) that represented the difference between the δ2H and a linear transform of the δ18O of a water sample. In the formula, a and b are the slope and intercept of the local meteoric water line. Lc-excess was used to determine the fractionation of the hydrological processes (Dansgaard 1964; Evaristo et al. 2015). Water that undergoes fractionation by evaporation has a negative lc-excess (Landwehr et al. 2014; Sprenger 2016).
Results
Variation characteristics of water levels
The burial depth of the groundwater ranged from 2.4 to 6.1 m in the river valley. The depth of the groundwater at G3 and G7 ranged from 1.3 to 2.4 m (with an average of 1.8 m) and 0.1 to 1.6 m (with an average of 0.7 m), respectively (Fig. 2). The water level of the river ranged from 3196.0 to 3198.5 m a.s.l., and the runoff ranged from 2.8 to 388.0 m3/s (Fig. 2). The variation in the water level of the groundwater was consistent with that of the river (Fig. 2). The variation in the water level could be divided into two periods: the flood period (June to October) and the dry period (November to May of the following year) (Fig. 2). The flood period coincided with the rain period and the thawing period, indicating that temperature and precipitation influenced the hydrological process in the basin (Li et al. 2018a, b). The water levels of the groundwater and river water had three obvious peaks in May, July and September (Fig. 2). The maximum peak of the water level occurred in July. The variation in the upstream (G7) groundwater level was greater than that downstream (G3). Moreover, the changes in water levels mainly depended on precipitation, especially continuous precipitation and extreme precipitation events (Fig. 2).
Composition of stable isotopes
The stable isotopes (δ18O and δ2H) in the precipitation, groundwater, river water and lake water are shown in Fig. 3. Precipitation had the maximum ranges of δ18O and δ2H, and the ranges in groundwater, river water and lake water were within the range of precipitation. The local meteoric water line (LMWL: δ2H = 7.99 δ18O + 12.54) and local evaporation line of the groundwater and surface water (LEL-Groundwater: δ2H = 5.72 δ18O – 4.67; LEL-River: δ2H = 6.27 δ18O – 0.58; LEL-Lake: δ2H = 4.44 δ18O – 2.93) were calculated by using the values of δ18O and δ2H (Fig. 3). The slopes of the LEL of the groundwater (5.72) and river water (6.27) were lower than that of the LMWL (7.99) and higher than that of the LEL of lake water (4.44). The slope of the LEL of groundwater was lower than that of the LEL of river water due to the large runoff of the Buha River (Li et al. 2018a, b) and the strong evaporation of soil water (Liu et al. 2022). The samples of lake water were located below and near the enriched end of the LMWL because of the evaporation of lake water over many years (Echegoyen et al. 2022). The monthly averages of the δ18O and lc-excess values in the groundwater ranged from − 8.57‰ to − 8.12‰ and 0.42‰ to 3.02‰, respectively (Fig. 2). The monthly averages of the δ18O and lc-excess values in the river water ranged from − 9.17‰ to − 7.99‰ and –0.08‰ to 2.77‰, respectively (Fig. 2). The variation in δ18O values of the groundwater was relatively small, and the variation in the δ18O values of the river water was relatively large (Fig. 2). The groundwater and river water had opposite changes in their δ18O and lc-excess values from March to July and the same changes in these values from July to February of the following year (Fig. 2).
Geochemical characteristics
The pH values of groundwater, river water and lake water ranged from 7.4 to 8.9, 8.2 to 9.3 and 9.1 to 9.3, respectively. The average contents of TDS in groundwater, river water and lake water were 690 mg/L, 394 mg/L and 12,010 mg/L, respectively. These results indicated that the groundwater was slightly alkaline and that the lake water was alkaline. The mean values of total hardness (TH) in the groundwater and river water were 349 mg/L and 220 mg/L, respectively. The TDS and TH of groundwater were relatively high because of the intense interactions between rocks and water (Li et al. 2021). The hydrochemical types and the Gibbs boomerang model of the groundwater, river water and lake water are shown in Figs. 4 and 5. The hydrochemical types of most of the groundwater, river water and lake water samples were Ca–Mg–HCO3, Ca–Mg–HCO3 and Na–Cl, respectively (Fig. 4). The hydrochemical types of some of the groundwater samples were Na–Mg–HCO3 (e.g., site G2), Ca–Cl–HCO3 (e.g., site G1) and Ca–Mg–Cl (e.g., site G3) (Fig. 4). The evolutionary paths of the groundwater, river water and lake water samples were from “Rock dominance” to “Ocean” (Fig. 5). Some of the groundwater samples had endmembers located between “Rock dominance” and “Ocean” (e.g., G1, G2 and G3) (Fig. 5).
Discussion
Factors affecting geochemical characteristics
All of the lake samples had “Ocean” endmembers (Fig. 5), indicating that the hydrochemical composition of lake water was primarily dominated by evaporation and crystallization (Gibbs 1970; Xiao et al. 2018). Most of the groundwater and river water samples had “Rock dominance” endmembers (Fig. 5), indicating that most groundwater and river water were primarily dominated by rock weathering (Cui and Li 2014; Gibbs 1970; Yuan et al. 2020). The ionic ratios in water samples can identify origin of ions and anthropogenic effects in the water (Chen et al. 2018). Most of the river water had an HCO3−: (Ca2+ + Mg2+) ratio between 1:1 and 1:2 (Fig. 6a), indicating that calcite and gypsum were dissolved in the river runoff (Yuan et al. 2020). River water samples in March had an HCO3−: (Ca2+ + Mg2+) ratio close to 2:1 (Fig. 6a), and the TDS of the river water was relatively high in March. These results indicated that dolomite dissolution was enhanced when the river runoff was low (Fig. 2). Most of the groundwater had an HCO3−: (Ca2+ + Mg2+) ratio between 1:1 and 2:1 (Fig. 6a), indicating that calcite and dolomite were dissolved in the groundwater runoff (Yuan et al. 2020). The ratios of HCO3−: (Ca2+ + Mg2+) in some groundwater samples (e.g., G1, G2 and G3) were greater than 1:2 (Fig. 6a), indicating an additional contribution of Ca2+ and/or Mg2+ from other sources and/or the deposition of carbon (Chen et al. 2020). The groundwater at G2 displayed an excess of Na+ over Cl− (Fig. 6b), implicating the contribution of cation exchange (McNeil et al. 2005). The groundwater at G1 had a Cl−: Na+ ratio close to 1:1 (Fig. 6b). By combining the results of Fig. 3 and Fig. 4, it was determined that the groundwater at G1 was affected by lake water (saltwater). The groundwater at G3 displayed an excess of Cl− over Na+ (Fig. 6b), and the value of NO3− in that groundwater ranged from 5.0 to 275.9 mg/L (with an average of 88.06 mg/L). These results indicated that the groundwater at G3 was affected by human activity (Li et al. 2021). The cations of groundwater tended to change from Ca2+ to Na+, and the cations of river water tended from Ca2+ to Mg2+ (Fig. 4). The anions of groundwater tended to change from HCO3− to Cl−, and the anions of river water tended to change from HCO3− to SO42− (Fig. 4). Therefore, the above results demonstrated that the intense evaporation of groundwater, cation exchange, saltwater intrusion and human activity affected the groundwater (Cui and Li 2014; Li et al. 2021; Xiao et al. 2012). This region has the same hydrochemical type, evolution process and geochemical control factors of groundwater as the closed basin in the Qinghai-Tibet Plateau and arid area of northwest China (Li et al. 2020; Tian et al. 2019; Xiao et al. 2018; Zhou et al. 2021). The anions of groundwater tended to change from HCO3− to Cl−/ SO42−, and the cations tended to change from Ca2+/Mg2+ to Na + /K+. Groundwater chemistry is controlled by mineral dissolution, cation exchange, evaporation and human activities (Li et al. 2020; Xiao et al. 2018).
Characteristics of groundwater and river response to precipitation
Most of the groundwater and river water samples were located above the middle of the LMWL (Fig. 3). The δ18O value of the groundwater was depleted, and its lc-excess value was relatively high from June to October (Fig. 2). Substantial precipitation and rain occurred during this time (Fig. 2). These results indicated that groundwater and river water were recharged by precipitation at high altitudes or large amounts of precipitation (Cui and Li 2014, 2015; Dotsika et al. 2010; Hughes and Crawford 2012). These results also indicated that the precipitation effect was more evident than the evaporation effect before infiltration into the ground and the evaporation of soil water and river water flows (Cui and Li 2014; Dotsika et al. 2010; Xiao et al. 2018). The δ18O value of the river water was enriched, and the lc-excess value of the river water was relatively low from June to October, indicating that the river water may be recharged by precipitation at low altitudes or evaporation of surface water, because the temperature was high from June to October in the study area (Li et al. 2018a, b; Hu et al. 2018). The obvious variations in the δ18O and lc-excess values in the groundwater and river water were closely related to the flood period and rain period (Fig. 2). According to previous studies, approximately 70–80% of the annual precipitation in the Qinghai Lake Basin occurs in summer and autumn (Hu et al. 2018). Therefore, the intensity-distribution of precipitation has significant control on the compositions of stable isotopes in groundwater and river water (Kalvans et al. 2020).
The water levels of the groundwater and rivers slightly increased in response to continuous precipitation (Green 1 in Fig. 2). The δ18O value of the groundwater was depleted and the lc-excess value increased. The δ18O value of the river water was depleted, and the lc-excess value of the groundwater decreased due to the recharge of the river water by meltwater. Because the δ18O value of the river ice was depleted upstream, it underwent intense evaporation during the freezing period. The water levels of the groundwater and rivers rapidly increased in response to the substantial amount of precipitation (Green 2 in Fig. 2). The δ18O value of the groundwater was enriched and the lc-excess value was decreased due to the groundwater recharge by soil meltwater. The δ18O value of river water was depleted, and the lc-excess value of the groundwater increased due to the recharge of the river water by precipitation at high altitudes. The groundwater and river water levels had large peaks in response to substantial and continuous precipitation (green bars 3, 4, 5 and 6 in Fig. 2). As the water level of the river rose, the pressure of the river on the groundwater increased on both sides of the river, the path of the groundwater increased. The δ18O value of the groundwater was depleted and the lc-excess value increased. The δ18O value of the river water was enriched (green bars 3 and 4 in Fig. 2) or depleted (green bars 5 and 6 in Fig. 2). The lc-excess value of the river water decreased (green bars 3 and 4 in Fig. 2) or increased (green bars 5 and 6 in Fig. 2). Therefore, there could be four possible reasons for the different characteristics of isotopes in the groundwater and river water in response to precipitation: recharging by meltwater, precipitation altitude and intensity, soil water or the interaction between groundwater and surface water (Kalvans et al. 2020). The hydrological processes of groundwater in the plateau basin are similar due to the unique environmental settings and special groundwater system (Chang et al. 2018; Tan et al. 2021). For example, (1) the values of δ18O and δ2H in groundwater are closed to river water, (2) groundwater has an obvious response to precipitation and meltwater, and (3) groundwater has the characteristic of rapid regeneration. Meanwhile, the recharge of groundwater in this study area is consistent with that of closed basin in northwest arid area (Xiao et al. 2018).
Interaction between groundwater and surface water
The values of δ18O and δ2H in the groundwater and river water were not significantly different (Fig. 3), indicating that the recharge sources of the groundwater and river water were the same (both recharged from local precipitation and upstream river water). These results indicated a strong hydrological connection between groundwater and river water in the river valley (Xu et al. 2017). Understanding the interaction between surface water and groundwater is crucial for clarifying the hydrological conditions in coupled water systems (Boyraz and Kazezyilmaz-Alhan 2021; Sophocleous 2002; Yang et al. 2017). A continuous interaction between surface water and groundwater occurs near stream and lake areas and affects the total water budget of the hydrological system (Bertrand et al. 2014; Boyraz and Kazezyilmaz-Alhan 2021; Winter et al. 1998).
In this study, the interactions between groundwater and surface water were investigated in the main stream (Fig. 7) and river estuary (Fig. 8). According to the δ18O, lc-excess and TDS values (Fig. 7), there can be two flow directions in the main stream: river flow to groundwater (from March to August) and groundwater flow to the river (from August to January in the next year). The δ18O, lc-excess and TDS values of the groundwater and river water were different in the same flow direction due to the complexity of the recharge sources. As the temperature rose from March to May, the ice on the river began to melt. The TDS and δ18O values in the groundwater and river water rapidly decreased (Blue 1 in Fig. 7). As precipitation increased from May to August, the levels of the groundwater and river water increased (Fig. 2). The lc-excess and TDS values increased and the δ18O value decreased (Yellow 1 in Fig. 7). The groundwater path increased and the source of groundwater was recharged by precipitation at high altitudes due to the increasing pressure of the river on the groundwater. The δ18O and TDS values of the groundwater and river water rapidly decreased and their lc-excess values rapidly increased from August to September (Blue 2 in Fig. 7). Because the water levels of the groundwater and river declined (Fig. 2) and the flow direction changed as groundwater flowed to the river, the sources of groundwater and river water were recharged by precipitation or river water at high altitudes. The δ18O and TDS values of the groundwater and river water rapidly increased and their lc-excess values rapidly decreased from September to November (Yellow 2 in Fig. 7), indicating that more soil water flowed into the river through the groundwater. The water levels of the groundwater and river declined after November (Fig. 2), and soil water continuously flowed into the river through the groundwater. However, the groundwater and river water were recharged by water at high altitudes when the soil water was scarce (Blue 3 in Fig. 7).
The interaction between groundwater and surface water was more complex in this river estuary because of three interactions: groundwater and river, lake and river, and lake and groundwater. The influence of lake water on river water was not obvious (Figs. 4, 5 and 8) because the river water sampling sites were relatively far from the lake or because the river had a large amount of runoff (Fig. 1). The influence of lake water on groundwater (G1 and G2) was obvious (Figs. 4, 5, 6 and 8). The flow directions of the river estuary may be the same as those of the main stream: river flowing to groundwater (from March to August) and groundwater flowing to the river (from August to January). However, the effect of lake water on groundwater was different from that of the main stream in the river estuary (Fig. 8). Although there was some flow from the groundwater to the river water in river estuaries, the groundwater was mainly recharged by groundwater runoff at high altitudes, and the river water was recharged by river water from upstream. Because the δ18O value in the groundwater was depleted compared with that in the river water, the TDS value in the groundwater was high compared with that in the river water (Fig. 8). The δ18O values of in the groundwater (G1 and G2) decreased, and the lc-excess and TDS values increased during the flood period (green in Fig. 8). The δ18O and TDS values of the groundwater (G2) increased in August (Fig. 8), and the level of groundwater declined in August (Fig. 2), indicating that the groundwater (G2) was affected by lake water. The δ18O and lc-excess values of the groundwater at G1 were similar to those of the groundwater at G2, but the TDS of the groundwater at G1 was higher than that of the groundwater at G2, indicating that the groundwater at G1 was largely affected by lake water. This may be because the groundwater at G1 was close to the lake, and the depth below the surface was shallow (Fig. 1). The variation level of the groundwater at G3 was similar to that of the groundwater at G7 (Fig. 2), but the δ18O, lc-excess and TDS values were different from those of other groundwater samples (Fig. 8) because the groundwater at G3 was affected by human activity (Figs. 4, 5, 6 and 8). The effect of human activities on groundwater-surface water interactions has been previously confirmed (Hancock 2002; Liu et al. 2015; Yang et al. 2017).
Changes in the water quality during the interaction between groundwater and surface water
Water is a necessary resource for human survival and development, and changes in water quality directly affect human health, so research on environmental quality impacts on water has attracted much attention (Lapworth et al. 2022; Ma et al. 2020; Ondrasek et al. 2021; Winter 2001). In this study, the interaction between surface water and groundwater had a certain impact on the water environment (Figs. 7 and 8). The TDS value of the river water increased after groundwater flowed into the river (Fig. 7), indicating that the water quality of the river water was degraded. Studies have found that high concentrations of dissolved organic carbon (DIC) occurred when DIC-enriched groundwater dominated river discharge (Stewart et al. 2022). The TDS value of the groundwater increased when the water levels of the river and groundwater increased (Figs. 2, 7 and 8). The groundwater path increased and the duration of the interaction between rocks and water increased. Therefore, the increasing pressure of the river on the groundwater will cause the deterioration of groundwater quality. The TDS value in the groundwater was higher than 1000 mg/L in the river estuary (Fig. 8), so the water quality of the groundwater should be focused because it had exceeded the national health standard (Chinese General Administration of Quality Supervision 2017). Moreover, the TDS value in the groundwater was higher in the river estuary than in the main stream, indicating that the quality of the groundwater was affected by the high ion concentrations and TDS content of the lake water. The phenomenon of water quality deterioration caused by saltwater intrusion is often reported in inland saltwater lakes and coastal zones (Akshitha et al. 2021; Li et al. 2016a, b). According to this study (Figs. 4, 5, 6 and 8) and previous studies (Li et al. 2021; Xiao et al. 2012), the quality of groundwater is affected by human activity. The contents of TDS of groundwater and river water increased from upstream to downstream in closed lake basins and mountain areas (Li et al. 2020; Xiao et al. 2018). The increases of TDS and NO3− are particularly prominent in the areas of intense natural variability (Chiogna et al. 2018; Li et al. 2018a, b; Longyang 2019) and human activity (Rose 2007; Zhang et al. 2017). In conclusion, the influence factors of water quality should be taken seriously.
Conclusions
This study investigated the compositions of stable isotopes, hydrochemical characteristics and water levels of different water bodies, and revealed the responses of groundwater and rivers to precipitation and the interaction between surface water and groundwater, and explored the influence of the interaction between groundwater and surface water on water quality. The LMWL (δ2H = 7.99 δ18O + 12.54) and LEL of the groundwater and surface water (LEL-Groundwater: δ2H = 5.72 δ18O–4.67; LEL-River: δ2H = 6.27 δ18O–0.58; LEL-Lake: δ2H = 4.44 δ18O–2.93) were calculated by using the values of δ18O and δ2H in different water bodies. The hydrochemical types of most of the groundwater, river water and lake water samples were Ca–Mg–HCO3, Ca–Mg–HCO3 and Na–Cl, respectively. The groundwater was slightly alkaline and the lake water was alkaline. The variation in the water level of the groundwater was consistent with that of the river, and the variation in the water level could be divided into two different periods: the flood period (June to October) and the dry period (November to May). There could be four possible reasons for the different characteristics of isotopes in the groundwater and river water in response to precipitation: recharging by meltwater, precipitation altitude and intensity, soil water or the interaction between groundwater and surface water. Our results also indicated that the water quality of the river water was degraded when the groundwater flowed into the river. The quality of the groundwater and river water was affected by the high ion concentrations and TDS content of the lake water. The increasing pressure of the river on the groundwater will cause the deterioration of groundwater quality. The quality of the groundwater was also affected by saltwater intrusion and human activity.
Data availability
The data used in this study are available from the corresponding author upon reasonable request.
References
Aeschbach-Hertig W, Gleeson T (2012) Regional strategies for the accelerating global problem of groundwater depletion. Nat Geosci 5(12):853–861. https://doi.org/10.1038/NGEO1617
Akshitha V, Balakrishna K, Udayashankar HN (2021) Assessment of hydrogeochemical characteristics and saltwater intrusion in selected coastal aquifers of southwestern India. Mar Pollut Bull 173:112989. https://doi.org/10.1016/j.marpolbul.2021.112989
Barnett TP, Adam JC, Lettenmaier DP (2005) Potential impacts of a warming climate on water availability in snow-dominated regions. Nature 438(7066):303–309. https://doi.org/10.1038/nature04141
Bertrand G, Siergieiev D, Ala-Aho P, Rossi PM (2014) Environmental tracers and indicators bringing together groundwater, surface water and groundwater-dependent ecosystems: importance of scale in choosing relevant tools. Environ Earth Sci 72(3):813–827. https://doi.org/10.1007/s12665-013-3005-8
Boyraz U, Kazezyilmaz-Alhan CM (2021) An analytical solution for groundwater flow incorporating the effect of water bodies with sloping surfaces. Hydrolog Sci J 66(7):1211–1221. https://doi.org/10.1080/02626667.2021.1925675
Chang QX, Ma R, Sun ZY, Zhou AG, Hu YL, Liu YG (2018) Using isotopic and geochemical tracers to determine the contribution of glacier-snow meltwater to streamflow in a partly glacierized Alpine-Gorge catchment in Northeastern Qinghai-Tibet plateau. J Geophys Res-Atmos 123(18):10037–10056. https://doi.org/10.1029/2018JD028683
Chen J, Wu H, Qian H, Li XY (2018) Challenges and prospects of sustainable groundwater management in an agricultural plain along the Silk Road Economic Belt, north-west China. Int J Water Resour Dev 34(3):354–368. https://doi.org/10.1080/07900627.2016.1238348
Chen J, Qian H, Gao YY, Wang HK, Zhang MS (2020) Insights into hydrological and hydrochemical processes in response to water replenishment for lakes in arid regions. J Hydrol 581:124386. https://doi.org/10.1016/j.jhydrol.2019.124386
Chinese General Administration of Quality Supervision (2017) Standard for groundwater quality. Standards Press of China, Beijing (in Chinese)
Chiogna G, Skrobanek P, Narany TS, Ludwig R, Stumpp C (2018) Effects of the 2017 drought on isotopic and geochemical gradients in the Adige catchment, Italy. Sci Total Environ 645:924–936. https://doi.org/10.1016/j.scitotenv.2018.07.176
Cui BL, Li XY (2014) Characteristics of stable isotope and hydrochemistry of the groundwater around Qinghai Lake NE Qinghai-Tibet plateau China. Environ Earth Sci 71(3):1159–1167. https://doi.org/10.1007/s12665-013-2520-y
Cui BL, Li XY (2015) Stable isotopes reveal sources of precipitation in the Qinghai Lake Basin of the northeastern Tibetan Plateau. Sci Total Environ 527–528:26–27. https://doi.org/10.1016/j.scitotenv.2015.04.105
Cui BL, Li DS, Jiang BF, Wang Y, Wang YX, Wang LS, Li XY (2021) Stable isotopes and chloride ion of precipitation events in the northeastern Tibetan Plateau. China J Mt Sci 18(4):834–846. https://doi.org/10.1007/s11629-020-6574-5
Dansgaard W (1964) Stable isotopes in precipitation. Tellus 16(4):436–468. https://doi.org/10.3402/tellusa.v16i4.8993
Dogramaci S, Skrzypek G, Dodson W, Grierson PF (2012) Stable isotope and hydrochemical evolution of groundwater in the semi-arid Hamersley Basin of subtropical northwest Australia. J Hydrol 475:281–293. https://doi.org/10.1016/j.jhydrol.2012.10.004
Dong HM, Song YG, Zhang MS (2019) Hydrological trend of Qinghai Lake over the last 60 years: driven by climate variations or human activities? J Water Clim Chang 10(3):524–534. https://doi.org/10.2166/wcc.2018.033
Dotsika E, Lykoudis S, Poutoukis D (2010) Spatial distribution of the isotopic composition of precipitation and spring water in Greece. Glob Planet Change 71(3–4):141–149. https://doi.org/10.1016/j.gloplacha.2009.10.007
Echegoyen CV, Campodonico VA, Lecomte KL, Jobbagy EG, Yaciuk PA, Sepulveda LD (2022) Surface-water/groundwater exchange in a sand dune lake in the Dry Pampean Plain, Argentina: stable isotopic evidence. Hydrogeol J 30(3):783–796. https://doi.org/10.1007/s10040-022-02449-w
Evaristo J, Jasechko S, McDonnell JJ (2015) Global separation of plant transpiration from groundwater and streamflow. Nature 525(7567):91–94. https://doi.org/10.1038/nature14983
Fan CY, Song CQ, Li WK, Liu K, Cheng J, Fu CS, Chen T, Ke LH, Wang JD (2021) What drives the rapid water-level recovery of the largest lake (Qinghai Lake) of China over the past half century? J Hydrol 593:125921. https://doi.org/10.1016/j.jhydrol.2020.125921
Feike T, Khor LY, Mamitimin Y, Ha N, Li L, Abdusalih N, Xiao HF, Doluschitz R (2017) Determination of cotton farmers’ irrigation water management in arid Northwestern China. Agric Water Manage 187:1–10. https://doi.org/10.1016/j.agwat.2017.03.012
Florke M, Kynast E, Barlund I, Eisner S, Wimmer F, Alcamo J (2013) Domestic and industrial water uses of the past 60 years as a mirror of socio-economic development: a global simulation study. Glob Environ Change-Human Policy Dimens 23(1):144–156. https://doi.org/10.1016/j.gloenvcha.2012.10.018
Foster S, MacDonald A (2014) The ‘water security’ dialogue: Why it needs to be better informed about groundwater. Hydrogeol J 22(7):1489–1492. https://doi.org/10.1007/s10040-014-1157-6
Frampton A, Painter SL, Destouni G (2013) Permafrost degradation and subsurface-flow changes caused by surface warming trends. Hydrogeol J 21(1):271–180. https://doi.org/10.1007/s10040-012-0938-z
Ge S, Mckenzie J, Voss C, Wu QB (2011) Exchange of groundwater and surface-water mediated by permafrost response to seasonal and long term air temperature variation. Geophys Res Lett 38(14):3138–3142. https://doi.org/10.1029/2011GL047911
Gibbs RJ (1970) Mechanisms controlling world water chemistry. Science 170(3962):1088–1090. https://doi.org/10.1126/science.170.3962.1088
Gibson JJ, Edwards TWD, Birks SJ (2005) Progress in isotope tracer hydrology in Canada. Hydrol Process 19:303–327. https://doi.org/10.1002/hyp.5766
Haddeland I, Heinke J, Biemans H, Eisner S, Florke M, Hanasaki N, Konzmann M, Ludwig F, Masaki Y, Schewe J (2014) Global water resources affected by human interventions and climate change. PNAS 111(9):3251–3256. https://doi.org/10.1073/pnas.1222475110
Hancock PJ (2002) Human impacts on the stream-groundwater exchange zone. Environ Manage 29(6):763–781. https://doi.org/10.1007/s00267-001-0064-5
Howells M, Hermann S, Welsch M, Bazilian M, Segerstorom R, Alfstad T, Gielen D, Rogner H, Fischer G, Van Velthuizen H, Wiberg D, Young C, Roehrl RA, Mueller A, Steduto P, Ramma I (2013) Integrated analysis of climate change, land-use, energy and water strategies. Nature Clim Change 3(7):621–626. https://doi.org/10.1038/NCLIMATE1789
Hu X, Li ZC, Li XY, Wang P, Zhao YD, Liu LY, Lu YL (2018) Soil macropore structure characterized by x-ray computed tomography under different land uses in the Qinghai Lake Watershed Qinghai-Tibet Plateau. Pedosphere 28(3):478–487. https://doi.org/10.1016/S1002-0160(17)60334-5
Hughes CE, Crawford J (2012) A new precipitation weighted method for determining the meteoric water line for hydrological applications demonstrated using Australian and global GNIP data. J Hydrol 464:344–351. https://doi.org/10.1016/j.jhydrol.2012.07.029
Immerzeel WW, van Beek LPH, Bierkens MFP (2010) Climate change will affect the Asian water towers. Science 328:1382–1385. https://doi.org/10.1126/science.1183188
Jia XY, O’Connor D, Hou DY, Jin YL, Li GH, Zheng CM, Ok YS, Tsang DCW, Lu J (2019) Groundwater depletion and contamination: spatial distribution of groundwater resources sustainability in China. Sci Total Environ 672:551–562. https://doi.org/10.1016/j.scitotenv.2019.03.457
Kalvans A, Delina A, Babre A, Popovs K (2020) An insight into water stable isotope signatures in temperate catchment. J Hydrol 582:124442. https://doi.org/10.1016/j.jhydrol.2019.124442
Landwehr JM, Coplen TB, Stewart DW (2014) Spatial, seasonal, and source variability in the stable oxygen and hydrogen isotopic composition of tap waters throughout the USA. Hydrol Process 28(21):5382–5422. https://doi.org/10.1002/hyp.10004
Landwehr JM, Coplen TB (2004) Line-conditioned excess: A new method for characterizing stable hydrogen and oxygen isotope ratios in hydrologic systems. In: International conference on isotopes in environmental studies. IAEA, Vienna.
Lapworth DJ, Boving TB, Kreamer DK, Kebede S, Smedley PL (2022) Groundwater quality: global threats, opportunities and realising the potential of groundwater. Sci Total Environ 811:152471. https://doi.org/10.1016/j.scitotenv.2021.152471
Li CC, Liu T, Xu S, Gao XB, Wang YX (2016a) The groundwater path increased and the duration of the interaction between rocks and water increased. Environ Earth Sci 75(5):370. https://doi.org/10.1007/s12665-016-5260-y
Li ZX, Qi F, Song Y, Wang QJ, Yang J, Li YG, Li JG, Guo XY (2016b) Stable isotope composition of precipitation in the south and north slopes of Wushaoling Mountain, northwestern China. Atmos Res 182:87–101. https://doi.org/10.1016/j.atmosres.2016.07.023
Li X, Yang X, Ma Y, Hu G, Hu X, Wu X, Wei J (2018a) Qinghai Lake Basin Critical Zone Observatory on the Qinghai-Tibet Plateau. Vadose Zone J 17(1):1–11. https://doi.org/10.2136/vzj2018.04.0069
Li ZH, Wang GC, Wang XS, Wan L, Shi ZM, Wanke HK, Uugulu S, Uahengo CI (2018b) Groundwater quality and associated hydrogeochemical processes in Northwest Namibia. J Geochem Explor 186:202–214. https://doi.org/10.1016/j.gexplo.2017.12.015
Li DL, Chen HY, Jia SF, Lv AF (2020) Possible hydrochemical processes influencing dissolved solids in surface water and groundwater of the Kaidu River Basin Northwest China. Water 12(2):467. https://doi.org/10.3390/w12020467
Li DS, Cui BL, Wang Y, Wang YX, Jiang BF (2021) Source and quality of groundwater surrounding the Qinghai Lake NE Qinghai-Tibet Plateau. Groundwater 59(2):245–255. https://doi.org/10.1111/gwat.13042
Liu F, Williams MW, Caine N (2004) Source waters and flow paths in an alpine catchment, Colorado front range United States. Water Resour Res 40(9):1–16. https://doi.org/10.1029/2004WR003076
Liu F, Song XF, Yang LH, Han DM, Zhang YH, Ma Y, Bu HM (2015) The role of anthropogenic and natural factors in shaping the geochemical evolution of groundwater in the Subei Lake basin, Ordos energy base, Northwestern China. Sci Total Environ 538:327–340. https://doi.org/10.1016/j.scitotenv.2015.08.057
Liu YE, Yu KK, Zhao YQ, Bao JC (2022) Impacts of climatic variation and human activity on runoff in Western China. Sustainability 14(2):942. https://doi.org/10.3390/su14020942
Longyang QQ (2019) Assessing the effects of climate change on water quality of plateau deep-water lake :a study case of Hongfeng Lake. Sci Total Environ 647:1518–1530. https://doi.org/10.1016/j.scitotenv.2018.08.031
Ma T, Sun S, Fu GT, Hall JW, Ni Y, He LH, Yi JW, Zhao N, Du YY, Pei T, Cheng WM, Song C, Fang CL, Zhou CH (2020) Pollution exacerbates China’s water scarcity and its regional inequality. Nat Commun 11(1):650. https://doi.org/10.1038/s41467-020-14532-5
Mariangela S, Forte G, Ducci D (2019) Identification of the natural background levels in the Phlaegrean fields groundwater body (Southern Italy). J Geochem Explor 200:181–192. https://doi.org/10.1016/j.gexplo.2019.02.007
Martinez JL, Raiber M, Cox ME (2015) Assessment of groundwater-surface water interaction using long-term hydrochemical data and isotope hydrology: headwaters of the Condamine River, Southeast Queensland, Australia. Sci Total Environ 536:499–516. https://doi.org/10.1016/j.scitotenv.2015.07.031
Mas-Pla J, Menció A (2019) Groundwater nitrate pollution and climate change: learnings from a water balance-based analysis of several aquifers in a western Mediterranean region (Catalonia). Environ Sci Pollut Res 26(3):2184–2202. https://doi.org/10.1007/s11356-018-1859-8
McNeil VH, Cox ME, Preda M (2005) Assessment of chemical water types and their spatial variation using multi-stage cluster analysis, Queensland. Australia. J Hydrol 310(1–4):181–200. https://doi.org/10.1016/j.jhydrol.2004.12.014
Ondrasek G, Begic HB, Romic D, Brkic Z, Husnjak S, Kovacic MB (2021) A novel LUMNAqSoP approach for prioritising groundwater monitoring stations for implementation of the Nitrates Directive. Environ Sci Eur 33(1):23. https://doi.org/10.1186/s12302-021-00467-1
Piper AM (1944) A graphic procedure in the geochemical interpretation of water-analyses. Eos Trans Amer Geophys Union 25(6):914–928. https://doi.org/10.1029/TR025i006p00914
Posthuma L, Munthe J, van Gils J, Altenburger R, Muller C, Slobodnik J, Brack W (2019) A holistic approach is key to protect water quality and monitor, assess and manage chemical pollution of European surface waters. Environ Sci Eur 31(1):67. https://doi.org/10.1186/s12302-019-0243-8
Raghavendra NS, Deka PC (2015) Sustainable development and management of groundwater resources in mining affected areas: a review. Procedia Earth and Planetary Sci 11:598–604. https://doi.org/10.1016/j.proeps.2015.06.061
Rodgers P, Soulsby C, Waldron S (2005) Stable isotope tracers as diagnostic tools in upscaling flow path understanding and residence time estimates in a mountainous mesoscale catchment. Hydrol Process 19:2291–2307. https://doi.org/10.1002/hyp.5677
Rose S (2007) The effects of urbanization on the hydrochemistry of base flow within the Chattahoochee River Basin (Georgia, USA). J Hydrol 341(1–2):42–54. https://doi.org/10.1016/j.jhydrol.2007.04.019
Somers LD, McKenzie JM (2020) A review of groundwater in high mountain environments. Wiley Interdisciplinary Reviews-Water 7(6):e1475. https://doi.org/10.1002/wat2.1475
Sophocleous M (2002) Interactions between groundwater and surface water: the state of the science. Hydrogeol J 10(1):52–67. https://doi.org/10.1007/s10040-002-0204-x
Soulsby C, Malcolm R, Helliwell R, Ferrier RC, Jenkins A (2000) Isotope hydrology of the Allt a’ Mharcaidh catchment, Cairngorms Scotland: implications for hydrological pathways and residence times. Hydrol Process 14(4):747–762
Sprenger M, Leistert H, Gimbel K, Weiler M (2016) Illuminating hydrological processes at the soil-vegetation-atmosphere interface with water stable isotopes. Rev Geophys 54(3):674–704. https://doi.org/10.1002/2015RG000515
Stewart B, Zhi W, Sadayappan K, Sterle G, Harpold A, Li L (2022) Soil CO2 controls short-term variation but climate regulates long-term mean of riverine inorganic carbon. Global Biogeochem Cy 36(8):e2022GB007351. https://doi.org/10.1029/2022GB007351
Tan HB, Zhang Y, Rao WB, Guo HY, Ta WQ, Lu SC, Cong PX (2021) Rapid groundwater circulation inferred from temporal water dynamics and isotopes in an arid system. Hydrol Process 35(6):e14225. https://doi.org/10.1002/hyp.14225
Tian Y, Yu CQ, Zha XJ, Gao X, Dai EF (2019) Hydrochemical characteristics and controlling factors of natural water in the border areas of the Qinghai-Tibet Plateau. J Geogr Sci 29(11):1876–1894. https://doi.org/10.1007/s11442-019-1994-y
Uhlenbrook S, Frey M, Leibundgut C, Maloszewski P (2002) Hydrograph separations in a mesoscale mountainous basin at event and seasonal timescales. Water Resour Res 38(6):31–14. https://doi.org/10.1029/2001WR000938
UN (United Nations) (2015) The Millennium Development Goals Report 2015.
Vorosmarty C, Green P, Salisbury J, Lammers RB (2000) Global water resources: vulnerability from climate change and population growth. Science 289:284–288. https://doi.org/10.1126/science.289.5477.284
Wada Y, Wisser D, Bierkens MFP (2014) Global modeling of withdrawal, allocation and consumptive use of surface water and groundwater resources. Earth System Dynamics 5(1):15–40. https://doi.org/10.5194/esd-5-15-2014
Wellman TP, Voss CI, Walvoord MA (2013) Impacts of climate, lake size, and supra-and sub-permafrost groundwater flow on lake-talik evolution, Yukon Flats, Alaska (USA). Hydrogeol J 21(1):281–298. https://doi.org/10.1007/s10040-012-0941-4
Winter TC (2001) Ground water and surface water: the linkage tightens, but challenges remain. Hydrol Process 15(18):3605–3606. https://doi.org/10.1002/hyp.504
Winter TC, Harvey JW, Franke OL, Alley WM (1998) Ground water and surface water: A single resource. Denver, Colorado: U.S. Geological Survey.
Xiao J, Jin ZD, Zhang F, Wang J (2012) Solute geochemistry and its sources of the groundwater’s in the Qinghai Lake Catchment, NW China. J Asian Earth Sci 52:21–30. https://doi.org/10.1016/j.jseaes.2012.02.006
Xiao Y, Shao JL, Frape SK, Cui YL, Dang XY, Wang SB, Ji YH (2018) Groundwater origin, flow regime and geochemical evolution in arid endorheic watersheds: a case study from the Qaidam Basin, northwestern China. Hydrol Earth Syst Sc 22(8):4381–4400. https://doi.org/10.5194/hess-22-4381-2018
Xu W, Su XS, Dai AX, Yang FT, Zhu PC, Huang Y (2017) Multi-tracer investigation of river and groundwater interactions: a case study in Nalenggele River basin, northwest China. Hydrogeol J 25(7):2015–2029. https://doi.org/10.1007/s10040-017-1606-0
Yang Z, Zhou YX, Wenninger J, Uhlenbrook S, Wang XS, Wan L (2017) Groundwater and surface-water interactions and impacts of human activities in the Hailiutu catchment, northwest China. Hydrogeol J 25(5):1341–1355. https://doi.org/10.1007/s10040-017-1541-0
Yuan RD, Wang M, Wang SQ, Song XF (2020) Water transfer imposes hydrochemical impacts on groundwater by altering the interaction of groundwater and surface water. J Hydrol 583:124617. https://doi.org/10.1016/j.jhydrol.2020.124617
Zaidi FK, Nazzal Y, Ahmed I, Naeem M, Jafri MK (2015) Identification of potential artificial groundwater recharge zones in Northwestern Saudi Arabia using GIS and Boolean logic. J Afr Earth Sci 111:156–169. https://doi.org/10.1016/j.jafrearsci.2015.07.008
Zhang QQ, Wang HM, Wang YC, Yang MN, Zhu L (2017) Groundwater quality assessment and pollution source apportionment in an intensely exploited region of northern China. Environ Sci Pollut Res 24(20):16639–16650. https://doi.org/10.1007/s11356-017-9114-2
Zhang L, Li PY, He XD (2021) Interactions between surface water and groundwater in selected tributaries of the Wei River (China) revealed by hydrochemistry and stable isotopes. Hum Ecol Risk Assess 28(1):79–99. https://doi.org/10.1080/10807039.2021.2016054
Zhou B, Wang HW, Zhang QQ (2021) Assessment of the evolution of groundwater chemistry and its controlling factors in the Huangshui river Basin of Northwestern China, using hydrochemistry and multivariate statistical techniques. Int J Env Res Pub He 17(14):7551. https://doi.org/10.3390/ijerph18147551
Acknowledgements
The study was supported by the National Natural Science Foundation of China (42177236), the Special Fund of Taishan Scholars Project, and the Science and Technology Support Plan for Youth Innovation of Colleges and Universities of Shandong (2019KJH009).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Ethical approval
The authors confirm that the work has been done according to ethical standards of scientific research.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Li, D., Cui, B., Zuo, F. et al. Hydrological characteristics and water quality change in mountain river valley on Qinghai-Tibet Plateau. Appl Water Sci 13, 104 (2023). https://doi.org/10.1007/s13201-023-01906-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13201-023-01906-5