Abstract
Poly(vinylidene fluoride) (PVDF) ultrafiltration (UF) membrane was prepared by vapor and non-solvent-induced phase separation (VNIPS) process, and the relationships between preparation conditions, phase separation behaviors and membrane structures were discussed. The phase diagram was generated by cloud point titration, the effects of environmental relative humidity, air exposure time and casting solution temperature on the structure and performance of the resulted membrane were investigated. The addition of polyvinylpyrrolidone (PVP) additives to the casting solution made the system thermodynamically unstable and led the turbidity curve shift toward the solvent/non-solvent axis. The experimental results showed that both average pore size and porosity increased with higher relative humidity. When the relative humidity increased from 35 to 85%, the mean pore size increased from 35 to 70 nm. The effect of exposure time on membrane structure was related to the air environment. It was found that under low temperature and low humidity conditions, exposure time had little effect on membrane structure. When the relative humidity was 75%, the mean pore size and distribution increased with the exposure time extension from 1.0 to 30 s. The mean pore size of the membrane could be reduced by reducing the relative humidity. When the temperature of the casting solution increased, the membrane pore size reached maximum at 80 °C, and the spherulite on the surface of the membrane decreased. The spherulite morphology almost disappeared when the temperature of the casting solution reached 120 °C. In addition, with the increase in the casting solution temperature, large pores appeared in the sub-layer of the membrane, which gradually widened the pore distribution, leading to the decrease in the tensile strength. The preparation condition was optimized as low temperature of casting solution, low humidity and low temperature of the environment. The fabrication process showed the potential for scaling-up production of the PVDF UF membrane by the VNIPS technique.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Poly(vinylidene fluoride) (PVDF) is one of the most popular ultrafiltration (UF) membrane materials because of its outstanding properties such as good chemical resistance, high thermal stability, antioxidation property and mechanical strength (Yabuno et al. 2020; Ilyas et al. 2020). It is mainly used in water treatment, membrane distillation (MD) and membrane bioreactor (MBR) (Cui et al. 2020; Zahid et al. 2018; Wei et al. 2018). The preparation of PVDF membrane started in the early 1980s (Kang and Cao 2014), and it can be processed by various methods such as phase inversion, melt drawing, sol–gel process and electrospinning (Zuo et al. 2019). Phase inversion is the most widely employed method which includes non-solvent-induced phase separation (NIPS) (Jung et al. 2016), thermally induced phase separation (TIPS) (Hu et al. 2016) and vapor-induced phase separation (VIPS), as well as the combination of these methods (Tsai et al. 2006; Fan et al. 2013a) depending on the mechanism.
NIPS is the most common method to prepare microfiltration (MF) and ultrafiltration (UF) membranes in industry. It was developed by Loeb and Sourirajan, at least three components are required: polymer, solvent, and non-solvent in the process. The membrane prepared by NIPS usually shows a typical asymmetric structure including interconnected bi-continuous network structure, finger-like structure, spherulite structure and sponge-like structure. Ahmad et al. (2020) developed a mathematical model to describe the phase inversion kinetics for the fabrication of polymeric membrane by NIPS process. The results revealed that the kinetics was controlled by the diffusion between solvent and non-solvent through porous membrane media. It has an adverse effect on membrane selectivity because of the difficulty to achieve the even distribution phase separation which can lead to a wide distribution of pore size (Ma et al. 2017; Kim et al. 2016). TIPS is commonly known for membranes preparation since proposed by Castro in the 1980s. TIPS has been rapidly developed and used to form MF and UF from a variety of crystalline amorphous polymers or semi-crystalline polymers (Liu et al. 2016; Zhang et al. 2015). TIPS requires high temperature to dissolve those polymers which are not soluble in common solvents at room temperature (Hassankiadeh et al. 2014). The surface properties of membranes prepared by TIPS, such as pore size and hydrophilicity, cannot be effectively adjusted by adding multifunctional additives like NIPS (Bottino et al. 1985). VIPS was first proposed by Zsigmondy and Bachmann (Zsigmondy and Bachmann 1918) and then developed further by Elford (Elford 1937). In recent years, VIPS has been applied for membrane preparation with superhydrophobic surface, specific morphologies or specific property. Xu et al. (2020) prepared a kind of antifouling poly(ether sulfone) (PES) composite membrane with bi-continuous porous structures and high fluxes by VIPS method. The results showed that the temperature of dissolution and vapor, the vapor relative humidity, the additive mass ratios and the exposure time remarkably influenced the membrane microstructures and the hydrophilicity. Compared with NIPS method, the phase separation that occurs during VIPS process is more stable, and the mass transfer rate (non-solvent inhalation and solvent extraction) is greatly reduced, which can effectively avoid macroporous defects. This method often combines with other processes to prepare porous membrane materials.
Combining VIPS and NIPS processes together, VNIPS is an innovative method for membrane preparation that can produce asymmetric polymer membranes with different features. VNIPS combines the advantages of VIPS and NIPS, and minimizes the disadvantages of membrane preparation of the two methods. For example, it reduces the finger-like pore structure and large hole defects on the membrane surface caused by double diffusion of VIPS method, and improves the repeatability of membrane preparation. The requirements of membrane preparation environment are more feasible than that of VIPS method, which is suitable for large-scale industrial application and can obtain polymeric membranes with controllable micro-structure and performance. The performance of membranes via VNIPS is often affected by several parameters such as the exposure time of nascent membrane in the humid atmosphere before immersing in coagulation bath, the state of the casting solution, conditions of preparation and air environment and so on. Ghandashtani et al. (2015) prepared PES MF membranes for the oil-in-water emulsion by VNIPS and concluded that the optimum preparation conditions were exposure time 33 s and humidity 80%, respectively, and the oil rejection was higher than 98%. Dehban et al. (2020) prepared the anti-fouling PPSU/PES/Nano silica composite UF membranes by VNIPS. The results showed that the permeability was mainly affected by the polymer concentration and the VIPS time. A VNIPS method using the double-layer casting process was explored by Fan et al. (2013b) who investigated the effect of VIPS time on the preparation of hydrophobic PVDF membrane for application in vacuum MD. It was observed that the longer exposure time from 0 to 5 min of membrane placed in saturated water vapor led to a higher permeating flux from 15.7 to 22.4 kg m−2 h−1.
In this paper, PVDF membrane was prepared by the combined VNIPS process with milder preparation conditions in phase separation. And the membrane preparation conditions including relative humidity, air exposure time and the temperature of casting solution were discussed as the essential phase separation parameters. It aimed to investigate the principle factors to control the micro-structure of PVDF membranes for improved performance as anti-fouling property and long-life service. These factors were found having significant effect on the chemical and physical properties of the membrane majorly for the pore dimension and distribution. The PVDF ultrafiltration membrane prepared by the optimal VNIPS technique can be widely applied in wastewater treatment, gas purification, food processing and other fields.
Experimental
Materials
Poly(vinylidene fluoride) (PVDF, FR904) was supplied by Shanghai 3F New Materials Co., Ltd. Polyvinylpyrrolidone (PVP, K30) and isopropanol (AR) were purchased from Sinopharm Group Co., Ltd. N, N-dimethylacetamide (DMAc) and glycerol (AR) was kindly provided by Shanghai Spectrum Biological Co., Ltd. Isobutanol (AR) was supplied from Shanghai Lingfeng Chemical Reagent Co., Ltd and Dextran (Mw: 10,000–65,000) was purchased from Sigma-Aldrich (Shanghai) Trading Co., Ltd. Deionized water was produced by Millipore pure water filter (Direct-Q3, American).
Phase diagram measurement
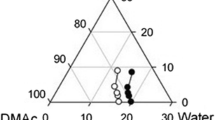
The construction of phase diagrams is crucial for analyzing polymer/solvent/additive/non-solvent interactions. The phase diagram of the PVDF/DMAc/additive/H2O system was generated by cloud point titration (Yeow et al. 2003). Polymer dope solutions in DMAc were prepared with PVDF concentrations of 22, 20, 18, and 15 wt% at 60 °C. The content of the PVP additive was always 5 wt%. Deionized water (non-solvent) was gradually added to the system to induce phase separation. For the gel-like solutions, the composition of the solution was determined at the gel phase separation point at the evaluated temperature. For solutions that became turbid, the solution composition was determined at the liquid–liquid phase separation point at the evaluated temperature.
PVDF membrane preparation
The flowchart of the PVDF membrane preparation process is shown in Fig. 1. The PVDF membranes were prepared via VNIPS process using a high-precision flat sheet membrane casting machine (HLKGM3125, China). Casting solution was prepared by mixing 20 wt% PVDF, 5 wt% PVP and 75 wt% DMAc in a flask, being heated at 60 °C and stirred at 250 r/min until a homogeneous doping solution was formed. Then, the solution was de-gassed at vacuum condition, followed by casting into flat sheet with a thickness of 200 μm by the membrane casting equipment. Consequently, it was exposed in humid air for a certain time. During this period, the composition of the casting solution on the membranes surface and aggregation structure of polymers would be influenced by factors such as the properties of the non-solvents, the temperature of casting solution, and the temperature and humidity of the atmosphere. Humidity would induce the exchange of water vapor and solvent and then the membrane was formed through phase separation (Caquineau et al. 2010) in a coagulation bath which was an aqueous solution of DMAc at room temperature. The membrane was then transferred to a bleaching bath with DI water to extract the remaining solvents. Finally, the membrane was soaked in an aqueous glycerol solution for 8 h before drying.
Characterization of the PVDF membranes
The microstructure of PVDF membranes was observed by the Field Emission Scanning Electron Microscope (FESEM, Hitachi S-4800, Japan). Before FESEM observation, PVDF membrane was dried in a vacuum oven under the temperature of 30 °C for one day. Then, it was sharply fractured into a small piece in liquid nitrogen and adhered to a conductive tape. The morphology of surface and cross section was observed after being coated with gold using an Ion Sputtering device (Hitachi MC-1000, Japan).
The pore size distribution of PVDF membranes was measured by Liquid–Liquid Displacement Porometry (LLDP, PSMA-10, Gaoqian functional Materials Co. Ltd., China) (Lee et al. 2013). The porosity of the PVDF membranes was tested by weight method (Zeng et al. 2018). Tensile strength is mechanical property of membranes, which was measured by a digital mechanical strength meter (SH-20, Wenzhou Shandu Instrument Co. Ltd., China) (Wang et al. 2020).
The permeability of PVDF membranes was evaluated by the pure water flux (PWF) measurement equipment as shown in Fig. 2. The membrane was pre-pressed for 15 min at 0.15 MPa, then tested for 10 min at 0.1 MPa. Each sample was tested for four times, and the average value was recorded as the PWF of the membrane under the current conditions. The PWF was calculated according to Eq. (1):
where Jw is the PWF (L m−2 h−1), v is the permeate volume (L) in a test period, Δt is the flux testing time (h); A is the effective area of membranes (m2).
Results and discussion
Determination of phase diagram
The ternary phase diagram in Fig. 3 shows the experimental data of the cloud point for PVDF/DMAc/water in the absence and presence of PVP at 60 ℃. The cloud point has been obtained as soon as local precipitation occurred, and the solution remained turbid. Otherwise, the non-solvent (water) was added again to the casting solution to achieve its cloud point. The gel formation usually shows imperceptible changes after a considerable time of agitation. The time after the addition of non-solvent (water) was extended to 3 h at the room temperature to verify visually if the gel state was maintained or not. The distance between the isothermal cloud point curve and the polymer/solvent axis reflected the degree of thermodynamic stability of the casting solution. Figure 3 shows that the addition of PVP additives into the casting solution broke the thermodynamic equilibrium of the system, making it thermodynamically unstable by shifting the turbidity curve towards the solvent/non-solvent axis. Therefore, less water quantity was required for PVDF precipitation in the quaternary PVDF/DMAc/PVP/water system than the ternary PVDF/DMAc/water system.
Effect of relative humidity
Relative humidity is one of the key factors influencing the phase separation behavior, and it usually fluctuates daily, climatically and seasonally. PVDF membranes were exposed for 5 s in the humidity chamber before being immersed in the coagulation bath, and the relative humidity of 35, 50, 60, 75, and 85% was investigated. Figure 4 shows the surface and cross section SEM images of the PVDF with various relative humidity. The casting solution temperature was 16 °C, the coagulation bath was 60 wt% DMAc solution at room temperature (20 °C). It was observed that when the relative humidity increased from 35 to 85%, the pore density on membrane surface increased and the pore size enlarged. The exposure time of 5 s in air for membranes enhanced the effect of the ambient relative humidity on the surface morphology of membranes. When the relative humidity increased, the density of water vapor in air increased, and therefore, more water vapor entered the membrane surface layer during the same exposure time and then the nuclei of phase-separated micronucleus was formed on the membrane surface layer (Shin et al. 2005). It increased the number of pores on the membrane surface, and at the same time, water vapor accumulated to form water droplets, leading to the enlargement of the membrane pore size (Liu et al. 2016). There was no obvious difference in the cross-sectional structures because of the negligible non-solvent volatilization in the casting solution under low preparation temperature. Besides, no change in the composition of membranes was shown except for the surface layer. Therefore, the increase in humidity had no effect on the cross-sectional structure of membranes.
Figure 5 shows the pore size distribution and the PWF changed with various relative humidity. As shown in Fig. 5a, the pore size at the maximum distribution of membranes gradually increased with the increase in the relative humidity. When relative humidity was 85%, the pore size at the maximum distribution of membranes was 75 nm, which was about twice than that at 35%. When the relative humidity decreased, the pore size distribution became narrower. The reason was the solvent in the membrane was exposed to air containing water vapor which was drawn onto the casting solution and resulted in nucleation of emulsion drops spontaneously. The pore size of membrane surface is strongly related to the content of water vapor in air (Wang et al. 2000). An increase in humidity can boost the content of water vapor in air, which then causes the nuclei of emulsion drops to grow, coalesce, and end up with the cellular surface pores (Wu et al. 2016). As shown in Fig. 5b, the PWF increased with the relative humidity, because the dense distribution of water vapor molecules under high humidity caused the increased number of emulsion nuclei. As a result, the cellular surface pore size enlarged. This was consistent with the results of SEM and pore size characterization.
Effect of air exposure time
In VNIPS process, membrane structure could be formed either in the humid air or in the coagulation bath. The time that the nascent membrane was exposed to humid air affected how complete the VIPS process was. It has a great influence on the final membrane structure (Su et al. 2009). The air exposure time of 1, 5, 10, 20, and 30 s was investigated with the comparative experiments under the conditions of relative humidity of 35 and 75% at room temperature (20 °C), respectively. The surface SEM images of PVDF membranes with different air exposure time under relative humidity of 35 and 75% are shown in Fig. 6. It can be seen that pore density gradually increased, the pore size gradually enlarged and more macropores appeared when the air exposure time changed from 1 to 30 s under relative humidity of 75%. Meanwhile, there was no obvious change under relative humidity of 35%. DMAc does not volatilize at 20 °C basically although it is a volatile solvent. Low solvent volatilization and low relative humidity of air would not affect the membrane surface. At high relative humidity of air the distribution of water vapor from the air into the initial casting solution surface layer became dense when the exposure time increased. And more cores of the phase-separated micronuclei were formed, resulting in the increase in pore numbers on the membrane surface eventually. Moreover, with the increase in the exposure time, water vapor continuously entered the surface layer of the initial casting solution, and part of the water droplets gathered with each other, which led to the enlargement of the pore size of membranes. When the water droplets became large, the surrounding solvents diffused to form poor-polymer phase, and these phases formed macropores structure as entering the coagulation bath. It resulted in the wider macropores size distribution of membranes. The prolongation of air exposure time did not present significant effects on the surface morphology of PVDF membranes under low relative humidity. It could be explained by the slight increase in number of surface pores, low solvent volatilization and narrow water vapor distribution in the air environment (Peng et al. 2012).
Figure 7 shows the effects of air exposure time on the pore size distribution and PWF under high humidity (75%). Figure 7a indicates that the pore size at the maximum distribution of the membranes enlarged, the macropore distribution became wider and Fig. 7b shows that the PWF increased from 283.1 to 518.7 L m−2 h−1 when the exposure time increased from 1.0 to 30 s. Similar to the discussion in Sect. 3.1, the effects of prolonging the exposure time were equivalent to increasing the air humidity in the case that the solvent of the casting solution was hardly volatile. Similarly, the water vapor in air entering the surface layer of initial casting solution increased and distributed densely, which resulted in the increase in pores number and the enlargement of the pore size. The aggregation of water vapor molecules entering the surface of the membrane was more obvious as extending the air exposure time under high relative humidity, which led to the wider distribution of macropores.
Effect of casting solution temperature
During the process of the polymeric membranes preparation, the viscosity and thermodynamic stability of casting solution changed with the temperature of the casting solution, while the kinetic and thermodynamic factors in the process of membrane preparation changed accordingly (ElSherbiny et al. 2015). At the same conditions of relative humidity, concentration of non-solvent additive and temperature, the viscosity of casting solution complies with the Arrhenius relation. The viscosity of casting solution decreased exponentially with the increased temperature. The increased temperature of casting solution enhanced the resistance of the polymer solution to non-solvents that the diffusivity also obeys the Arrhenius law (Bchetnia et al. 2004). It indicates that the temperature of the casting solution is an important factor to affect the formation of PVDF membranes. Combining the properties of the membrane material and solvents, temperatures of casting solution (20, 60, 80, 100, and 120 ℃) were investigated in experiment and the temperature of casting blade was consistent with casting solution. The remaining conditions of membrane preparation were as follows: the air exposure time was 1.0 s, the relative humidity was 35%, and the coagulation bath was 60 wt% DMAc aqueous solution at room temperature.
The surface and cross section SEM images of as-prepared membranes at different temperatures of the casting solution are shown in Fig. 8. It can be seen from the surface SEM images that the number of spherulites on the membrane surface decreased with the increase in the casting solution temperature. The spherulite morphology on the surface of membranes almost disappeared when the temperature of the casting solution reached 120 °C which indicated that solid–liquid phase separation was shifted to liquid–liquid phase separation. The reason for this phenomenon was the thermodynamic stability of the casting solution increased with the increasing temperature. It induced that the liquid–liquid phase separation became main trend, meanwhile the formation of spherulites on the surface of membranes decreased. There were still very few spherulites on the surface of membranes at 120 °C, which was due to the large temperature difference between the coagulation bath and the casting solution. As a result, solid–liquid phase separation still occurred on the membrane surface. The cross section SEM images showed sponge-like structure when the casting solution was 20 °C, and macropores began to appear in the sublayer area near the outer skin layer when the temperature of casting solution increased. Moreover, the macropores enlarged, the distribution of macropores widened, and the position became closer to the skin layer. This was because the viscosity decreased when the temperature of casting solution increased, which accelerated both the rate of non-solvent entered the casting solution and the rate of solvents entered the coagulation bath during the membrane preparation process. Hence, the separation layer of membranes formed rapidly and limited the exchange rate of solvents and non-solvents in the sublayer region (Boom et al. 1992).
Figure 9 shows the pore size distribution of PVDF membranes prepared with different casting solution temperature. The results indicated that the pore size at the maximum distribution enlarged firstly and then decreased when the temperature of casting solution increased, and the pore size at the maximum distribution was about 100 nm at 80 °C. The mean pore size increased with higher casting solution temperature. On the one hand, the decrease in viscosity and the increase in temperature shortened the time of phase separation. On the other hand, when casting solution temperature increased, its stability improved. The transition of the gel phase required an environment of higher non-solvent concentration, so that the content of the non-solvent increased when it reached an unsteady state. At the same time, the amount of solvent volatilization before the membrane entering the coagulation bath increased when the temperature of the casting solution increased. When the temperature was higher than the volatilization temperature of the solvents, the amount of the solvent volatilization could not be ignored, which increased the concentration of the polymers in membranes, especially on the upper surface layer of membranes, leading to the decrease in the pore size of the membrane surface.
The effect of the casting solution temperature on the PWF, porosity and tensile strength is shown in Table 1. It can be seen that the porosity of membranes slightly increased with the high casting solution temperature. The increased temperature of casting solution enlarged the size of macropores in the sublayer region under the membrane skin layer, and widened the distribution along the membrane thickness direction, then, the porosity increased. The PWF was related to the membrane pore size and porosity. The change of porosity was not obvious, so PWF was mainly related to the pore size. The PWF of membranes enlarged firstly and then decreased which was corresponding to the pore size and the PWF was 411.6 L m−2 h−1 when the temperature of casting solution was 80 °C. The macropores in the sublayer were larger with a wider distribution on the surface layer of membranes, made the internal structure of membranes became loose and the connectivity between the molecular chains inside the membrane could be weakened, which decreased the tensile strength of the membrane.
Conclusion
PVDF UF membrane was prepared by VNIPS process. By adding the PVP additives into the casting solution the turbidity curve moves toward the solvent/non-solvent axis. Both the increase in air relative humidity and air exposure time (except for low humidity) could increase the amount of water vapor entering the surface of the nascent membranes. As a result, it could enlarge the mean pore size and widen the macropores distribution. The temperature of casting solution presented a significant effect on the structure and properties of the as-prepared PVDF membranes. The increase in temperature accelerated the phase separation and the solvent volatilization rate on the membrane surface. The pore size enlarged firstly and then decreased with high temperature, and the mean pore size reached the maximum about 100 nm at 80 °C. With the increase in the temperature of casting solution, macropores began to appear in the sublayer whose distribution was widened along the direction of membrane thickness gradually.
References
Ahmad T, Guria C, Mandal A (2020) Kinetic modeling and simulation of non-solvent induced phase separation: Immersion precipitation of PVC-based casting solution in a finite salt coagulation bath. Polymer 199:122527
Bchetnia A, Souissi M, Rebey A, Jani BE (2004) Diffusion of vanadium in GaAs. J Cryst Growth 270:376–379
Boom RM, Wienk IM, Van Boomgaard Th., Smolders CA (1992) Microstructures in phase inversion membranes. Part 2. The role of a polymeric additive. J Membrane Sci 73:277–292.
Bottino A, Capannelli G, Munari S (1985) Effect of coagulation medium on properties of sulfonated polyvinylidene fluoride membranes. J Appl Polym Sci 30:3009–3022
Caquineau H, Menut P, Deratani A, Dupuy C (2010) Influence of the relative humidity on film formation by vapor induced phase separation. Polym Eng Sci 43:798–808
Cui ZL, Pan J, Wang ZH, Frappa M, Drioli E, Macedonio F (2020) Hyflon/PVDF membranes prepared by NIPS and TIPS: comparison in MD performance. Sep Purif Technol 247:116992
Dehban A, Kargari A, Ashtiani FZ (2020) Preparation and optimization of antifouling PPSU/PES/SiO2 nanocomposite ultrafiltration membranes by VIPS-NIPS technique. J Ind Eng Chem 88:292–311
Elford WJ (1937) Principles governing the preparation of membranes having graded porosities, The properties of "gradocol" membranes as ultrafilters. Trans Faraday Soc 33:1094–1104.
ElSherbiny IMA, Ghannam R, Khalil ASG, Ulbricht M (2015) Isotropic macroporous polyethersulfone membranes as competitive supports for high performance polyamide desalination membranes. J Membrane Sci 493:782–793.
Fan H, Peng Y, Li Z, Ping C, Qi J (2013a) Preparation and characterization of hydrophobic PVDF membranes by vapor-induced phase separation and application in vacuum membrane distillation. J Polym Res 134:1–15
Fan H, Peng Y, Li Z, Chen P, Jiang Q, Wang S (2013b) Preparation and characterization of hydrophobic PVDF membranes by vapor-induced phase separation and application in vacuum membrane distillation. J Polym Res 20:1–15
Ghandashtani MB, Ashtiani FZ, Karimi M, Fouladitajar A (2015) A novel approach to fabricate high performance nano-SiO2 embedded PES membranes for microfiltration of oil-in-water emulsion. Appl Surf Sci 349:393–402
Hassankiadeh NT, Cui ZL, Kim JH, Shin DW, Sanguineti A, Arcella V, Lee YM, Drioli E (2014) PVDF hollow fiber membranes prepared from green diluent via thermally induced phase separation: effect of PVDF molecular weight. J Membr Sci 471:237–246
Hu NG, Xiao TH, Cai XH, Ding LN, Fu YH, Xing Y (2016) Preparation and characterization of hydrophilically modified PVDF membranes by a novel nonsolvent thermally induced phase separation method. Membranes 18:47
Ilyas A, Mertens M, Oyaert S, Vankelecom IFJ (2020) Synthesis of patterned PVDF ultrafltration membranes: spray-modifed non-solvent induced phase separation. J Membr Sci 612:118383
Jung JT, Kim JF, Wang HH, Nicolo ED, Drioli E, Lee YM (2016) Understanding the non-solvent induced phase separation (NIPS) effect during the fabrication of microporous PVDF membranes via thermally induced phase separation (TIPS). J Membr Sci 514:250–263
Kang GD, Cao YM (2014) Application and modification of poly(vinylidene fluoride) (PVDF) membranes—a review. J Membr Sci 463:145–165
Kim JF, Kim JH, Drioli E, Lee YM (2016) Thermally-induced phase separation (TIPS) and electrospinning methods for emerging membrane applications: a review. AIChE J 62:461–490
Lee S, Dilaver M, Park P, Kim J (2013) Comparative analysis of fouling characteristics of ceramic and polymeric microfiltration membranes using filtration models. J Membr Sci 432:97–105
Liu M, Liu S, Xu Z, Wei Y, Yang H (2016) Formation of microporous polymeric membranes via thermally induced phase separation: a review. Front Chem Sci Eng 10:57–75
Ma B, Yang J, Sun QS, Jakpa W, Hou XL, Yang YQ (2017) Influence of cellulose/[Bmim]Cl solution on the properties of fabricated NIPS PVDF membranes. J Mater Sci 52:9946–9957
Peng YL, Fan HW, Dong YJ, Song YN, Han H (2012) Effects of exposure time on variations in the structure and hydrophobicity of polyvinylidene fluoride membranes prepared via vapor-induced phase separation. Appl Surf Sci 258:7872–7881
Shin SJ, Kim JP, Kim HJ, Jeon JH, Min BR (2005) Preparation and characterization of polyethersulfone microfiltration membranes by a 2-methoxyethanol additive. Desalination 186:1–10
Su YS, Kuo CY, Wang DM, Lai JY, Deratani A, Pochat C, Bouyer D (2009) Interplay of mass transfer, phase separation, and membrane morphology in vapor-induced phase separation. J Membr Sci 338:17–28
Tsai HA, Kuo CY, Lin JH, Wang DM, Deratani A, Pochat-Bohatier C, Lee KR, Lai JY (2006) Morphology control of polysulfone hollow fiber membranes via water vapor induced phase separation. J Membr Sci 278:390–400
Wang XZ, Li XG, Yue J, Cheng YM, Xu K, Wang Q, Fan F, Wang ZH, Cui ZL (2020) Fabrication of poly(vinylidene fluoride) membrane via thermally induced phase separation using ionic liquid as green diluent. Chin J Chem Eng 28:1415–1423
Wang DM, Wu, TT, Lin FC, Hou JY, Lai JY (2000) A novel method for controlling the surface morphology of polymeric membranes. J Membrane Sci 169:39–51.
Wei CJ, Dai FY, Lin LG, An AH, He Y, Chen X, Chen L, Zhao YP (2018) Simplified and robust adhesive-free superhydrophobic SiO2-decorated PVDF membranes for efficient oil/water separation. J Membr Sci 555:220–228
Wu DZ, Zhao L, Vakharia VK, Salim W, Winston Ho WS (2016) Synthesis and characterization of nanoporous polyethersulfone membrane as support for composite membrane in CO2 separation: From lab to pilot scale. J Membrane Sci 510:58–71.
Xu MH, Xie R, Ju XJ, Wang W, Liu Z, Chu LY (2020) Antifouling membranes with bi-continuous porous structures and high fluxes prepared by vapor-induced phase separation. J Membr Sci 611:118256
Yabuno Y, Mihara K, Miyagawa N, Komatsu K, Nakagawa K, Shintani T, Matsuyama H, Yoshioka T (2020) Preparation of polyamide-PVDF composite hollow fiber membranes with well-developed interconnected bicontinuous structure using high-temperature rapid NIPS for forward osmosis. J Membr Sci 612:118468
Yeow ML, Liu YT, Li K (2003) Isothermal phase diagrams and phase-inversion behavior of poly(vinylidene fluoride)/solvents/additives/water systems. Journal of Applied Polymerence 90:2150–2155
Zahid M, Rashid A, Akram S, Rehan ZA, Razzaq W (2018) A comprehensive review on polymeric nano-composite membranes for water treatment. Jf Membrane Sci Technol 8:1–20
Zeng KL, Zhou J, Cui ZL, Zhou Y, Shi C, Wang XZ, Zhou LY, Ding XB, Wang ZH, Drioli E (2018) Insight into fouling behavior of PVDF hollow fiber membranes caused by dextran with different pore size distributions. Chin J Chem Eng 2:268–277
Zhang ZC, Guo CG, Lv JL (2015) Tributyl citrate as diluent for preparation of PVDF porous membrane via thermally induced phase separation. Polym Polym Compos 23:175–180
Zsigmondy R, Bachmann W (1918) Uber neue filter. Anorg u Allgem Chem 103:119–128
Zuo JH, Li ZK, Wei C, Yan X, Chen Y, Lang WZ (2019) Fine tuning the pore size and permeation performances of thermally induced phase separation (TIPS)-prepared PVDF membranes with saline water as quenching bath. J Membr Sci 577:79–90
Acknowledgements
All authors would like to express their appreciation for the National Natural Science Foundation of China (No. 21921006) and the Jiangsu Provincial Department of Human Resources and Social Security (No. JNHB-036).
Funding
This study was funded by the National Natural Science Foundation of China (No. 21921006) and the Jiangsu Provincial Department of Human Resources and Social Security (No. JNHB-036).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
All authors certify that they have no affiliations with or involvement in any organization or entity with any financial interest or non-financial interest in the subject matter or materials discussed in this paper.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Chen, M., Sun, Q., Zhou, Y. et al. Preparation of PVDF membrane via synergistically vapor and non-solvent-induced phase separation. Appl Water Sci 12, 161 (2022). https://doi.org/10.1007/s13201-022-01683-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13201-022-01683-7