Abstract
The ground water quality of Okobo Local Government Area was investigated. Sixteen boreholes (BHs) water samples were collected from four zones (Okopedi, Ekeya, Ukwong and Okiuso) in Okobo. Standard analytical procedures were used to analyze the physicochemical, bacteriological and heavy metal parameters in the water samples and the results compared to Nigerian standard for drinking water quality (NSDWQ). some physicochemical parameters investigated were within the acceptable limits set by NSDWQ except pH (5.99 ± 0.37), DO (0.31 ± 0.06) mg/L, BOD5 (6.26 ± 0.4) mg/L and Nitrate (62.53 ± 5.96) mg/L. Bacteriological parameter like fecal coliform (128.69 ± 31.40) MPN/100 mL and total coliform (287.63 ± 40.31) MPN/100 mL were also above the limits set by NSDWQ implying organic pollution due to fecal contamination. Heavy metals were also within the acceptable limit except Lead (0.1 ± 0.1) mg/L, Chromium (0.4 ± 0.2) mg/L, and Manganese (0.16 ± 0.2) mg/L which were slightly above acceptable limits in all the zones. Water quality index calculation results grouped the BHs into; BH7 (26–50) very good; BH1, BH3, BH4, BH8, BH11, BH14, and BH16 (51–75) poor; BH2, BH5, BH6, BH9, BH12, BH13 and BH15 (76–100) very poor and BH 10(> 100) unsuitable for drinking. Pearson coefficient correlation, principal component analysis (PCA) and cluster analysis (CA) were used to establish interrelationship among the parameters, common sources of the pollutants and grouping of the BHs affected by these pollutants. PCA extracted six principal components (PCs) from the investigated parameters in the BHs, with sources of pollution either natural mineral or anthropogenic source. CA grouped all the sixteen BHs investigated into three clusters with various levels of contamination from pollutant sources. Consequently, the polluted BHs require treatment using high test hypochlorite (HTH) as the pollutant common to all the BHs is mostly bacterial pollutant; moreover, BHs should be sited 15 m away from septic tank or latrine to reduce contamination from coliform.
Similar content being viewed by others
Introduction
Water which is an essential compound in which life depends is derived mainly from sources like surface water; rain water and ground water. (Abdullahi et al. 2020; Ighalo and Adeniyi 2020).
When required for domestic consumption, it should posses a high degree of purity. Some natural sources of water like ground water is expected to be less contaminated, however its polarity and hydrogen bond makes water able to dissolves, absorbs, adsorbs or suspends impurities, (Ajala et al. 2020) therefore water from natural sources could get contaminants from natural and anthropogenic sources from its surrounding.
Recently, heavy metals contamination in ground water has been considered a serious environmental issue (Ighalo et al. 2021). These metals are found in groundwater in soluble form (Essumang et al. 2011).Their presence in water could either come from eroded mineral within sediments, draining of mineral deposits and volcanic eruption residues or from human activities like solid or liquid discharged from industrial or domestic processes. (Essumang et al. 2011).
Bacteriological contaminants in borehole water could be coliform, protozoan and viruses. These microorganisms could be vectors for water borne diseases such as dysentery, typhoid fever, cholera and other illness when such water is used domestically (Udoh et al. 2021). Human and animal wastes are major sources of bacterial contaminants in ground water. Sources of bacterial contamination are run-off from land use for rearing of animals, site used for manure preparation and seepage from poorly constructed sewage disposal facility. Pathogens from here can diffuse into the borehole water that do not have water tight casings.
Recently, multivariate statistical analyses like principal component analysis(PCA), cluster analysis(CA) and water quality index analysis(WQI) has been successfully used in water quality assessment studies (David et al. 2020), using these methods, data generated can be simplified and organized to yield useful information for decision making. (Amadi 2011; Kaiser 1985; Mohammed et al. 2016).
Borehole water is the major source of drinking water in the study area with limited information on the ground water quality. This research is tailored to assess the ground water quality in the area under consideration, evaluates the portability of the boreholes in terms of WQI, inform borehole water owners on the health implications associated with consumption of contaminated borehole water, recommends factors to be considered before sitting of BH and BH water treatment procedures.
Materials and methods
Study area
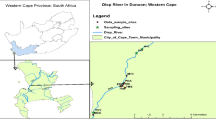
The study area is Okobo Local Government Area which is located in latitude 4° 4′ 59.98″N and longitude 7° 50′ 59.99′′ E. It is bounded on the west by Oron, North by Urue Ofong Oruko and Esit Eket, south by Uruan and East by Nsit Atai. Politically, Okobo is divided into seven districts namely: Eta, Odu, Egbuhu, Okiuso, Atabong, Ibighi, Ukwong with 65 villages. Okopedi is the headquarter. The major occupations of Okobo people are subsistence farming, fishing and petty trading. For spread of sampling points, the seven districts were divided into four zones in which four BHs were randomly selected from four different villages in each zone. Table 1 and Fig. 1 shows the coordinates the sampling points and map of the study area.
Sample collection and preservation
Samples were obtained from sixteen (16) BHs. The sample containers were pre-cleaned with acetone, rinsed with distilled water and kept in a dust free enclosure (American society for testing and materials (ASTM) (2016). Three replicate samples were obtained at each BH. Physicochemical parameter and metals samples were preserved in a two liter polyethene bottle. Sample for DO, BOD5 and bacteriological analysis were kept in glass bottle. Sample was allowed to flow from the tap for ten minutes before collecting for physicochemical parameters. For metals analysis, the sample collected was dosed with 1 mL of 2 M nitric acid. To obtained sample for DO, BOD5 and bacteriological analysis, pre-heating of the water tap with hot flame was required to eliminate possible bacteria present in the mouth of the tap and then the water was allowed to flow for five minutes before samples were obtained. All the samples were properly labeled, stored in an iced insulated container and transported to the laboratory for analysis. (USEPA 2002).
Physicochemical parameter analysis
Physical parameters like Temperature, pH, conductivity, salinity, turbidity and dissolved oxygen were all done in-situ. Temperature was measured using mercury in glass thermometer, pH using HACH SESSION+ digital pH meter, turbidity using HACH 20100N turbidity meter, conductivity, salinity, dissolved oxygen and total dissolved solid were measured using HACH 20100N conductivity meter. Colour was measured using LOVIBORD comparator and BOD5 was measured using HACH 20100N conductivity meter after five days. Heavy metals and bacteriological parameters were evaluated following standard procedures as describe in (Marcovecchio et al. 2007; ASTM 2016).
Statistical data analysis
Multivariate and descriptive statistical analysis was applied on water parameter data. SPSS IBM version 23 was used for Pearson coefficient correlation, PCA and CA. Pearson correlation was used to determine interrelationship exiting in the physical parameters, PCA was used to determine major pollution sources while CA was used to determine BHs with similar pollutants (Ugochukwu et al. 2021; Wu et al. 2005).
Water quality index
This index was used to determine portability of water from the selected water parameters. The WQI was calculated using standards recommended by NSDWQ. Weighted Arithmetic index method (Asibor and Ofuya 2019) was applied for the determination of WQI. Water quality rating (qa) was calculated using the expression in Eq. (1)
where (qa) represents the water quality rating of ath parameter, Va represents the measured value of the ath parameter at a given water sampling station, Sa represents the standard allowed value of the nth parameter and Vo represents an ideal value of nth parameter in pure water (0 for all other parameters except the parameters pH and Dissolve oxygen 7.0 and 14.6 mg/L respectively; (Asibor and Ofuya 2019). The unit weight (wa) was calculated by a value inversely proportional to the recommended standard value Sa corresponding to ath parameter Eq. (2).
K is the proportionality constant given in Eq. (3)
WQI is thus evaluated in Eq. (4)
Water quality index classification is shown in Table 2 (Chatterrji and Raziuddin 2002).
Results and discussion
Physicochemical parameters
Physicochemical, bacteriological and heavy metals concentration of BHs in the study area were investigated and results obtained were compared with NSDWQ Table 3a and b. A descriptive statistical analysis of the measured parameters is shown in Table 4.
The temperature range in the study area was from 27 °C to 29 °C with BH1 and BH10 having the lowest temperature while BH3, BH8, BH14 and BH15 had the highest temperature. The mean temperature was 28.08 ± 0.6 °C. This mean temperature for ground water was also reported by Adetinuke et al. (2016) in the assessment of ground water in BH in Lagos. The temperatures were in acceptable range as specified by NSDWQ.
pH defines the hydroxonium ion concentration in solution which expresses the degree of acidity and alkalinity. In the study area, the pH ranged from 5.20 to 6.70 and the mean pH was 5.99 ± 0.37. BH1 had the lowest pH of 5.2 while BH14 had the highest pH of 6.7. Except BH10 which had pH of 6.6, others had pH lower than the acceptable values specified by NSDWQ. The limit set by NSDWQ is ranged (6.5–8.5) (Standard organization of Nigeria, SON, 2015). This implies that the ground water in Okobo is slightly acidic. This trend was also reported by (Paschke et al. 2016). Similar observation was also reported by Enyoh et al. (2018). The possible reasons for the acidic level maybe as a result of breaking down of organic waste from human, animal waste and organic vegetation, or leaching of minerals into the BH water from mineral rich-rock (Enyoh et al. 2018).
Electrical conductivity (EC) in water is the ability of the water to conduct electricity due to the presence of dissolved mineral salts, total suspended solid, salinity and leaching of minerals into the water by mineral rich rocks. EC in the study area ranged from 50.0 µs/cm to 70.30 µs/cm. BH2 had the lowest EC value of 50.0 µs/cm while BH6 had the highest value of 70.30 µs/cm. The mean EC value was 61.76 ± 7.1 µs/cm. The measured EC values were all within the acceptable limit of 1000 µs/cm.
The value of colour obtained from the BHs water was 3 Hazen units. This value was within the NSDWQ acceptable unit of 15 Hezan units.
Turbidity is the level of cloudiness of water sample due to the presence of suspended particles. High turbidity value indicates the abundance of pathogens. Turbidity values were observed to be from 0.35 to 0.72 NTU. The mean value of turbidity in the study area was 0.505 ± 0.11 NTU. The mean turbidity value was in the specified range of 5 NTU.
Salinity is the amount of the salt content in water. The salts are mostly soluble chlorides and sulphates. High level of salinity in ground water is mostly due to seawater intrusion into the ground water. In the study area, mean salinity value was 42.03 ± 5.08 mg/L. BH4 had the highest salinity value of 48.4 mg/L while BH2 with value 33.2 mg/L was recorded the lowest. However, these values were within than the acceptable limits of 250 mg/L. From these results, it can be concluded that there is no seawater intrusion in the area under study.
Total hardness (TH), is the amount of soluble magnesium or calcium carbonates, bicarbonates or both present in water. The total hardness is the reflection of the amount of magnesium and calcium carbonates or bicarbonates or both in the water. Total hardness in all the BHs ranged from 26.3 mg/L to 50.20 mg/L. However, mean TH of the study area was 36.34 ± 9.03 mg/L. This value is within the acceptable limits of < 150 mg/L as set by NSDWQ. World Health Organization (WHO2004), classifies water with calcium carbonate level (0–60 mg/L) as soft water, therefore considering that observed values of TH, it can be concluded that the water in the study area is soft.
Total dissolved solid (TDS) is the amount of total suspended solids and dissolved minerals presence in water. In the area under review, the measured TDS values were from 33.50 mg/L to 47.10 mg/L. The acceptable limit set by the regulatory body is 500 mg/L. Hence TDS values in the study area are within acceptable limits.
Alkalinity is the degree of buffering of acidity in water. Alkalinity in water is due to the presence of carbonate ions CO32−, bicarbonates HCO3−, hydroxides OH−( The mean value from the study area was 88.91 ± 13.06 mg/L. Alkalinity values from the study area was within the acceptable limit of 150 mg/L set NSDWQ.
Dissolved oxygen (DO), shows concentration of oxygen gas that dissolved in water. In the area under consideration, the measured DO ranged from 0.20 mg/L to 0.44 mg/L. BH4 had the lowest value while BH1 has the highest. Mean DO value of 0.313 ± 0.056 mg/L of the study area was below than the specified limit of 5 mg/L. Low value of DO may be associated with high level of organic waste decomposition, high bacteria activity and bacterial contamination (Elemile et al. 2019).
Biological oxygen demand (BOD5) is the amount of oxygen needed for bacterial metabolic activities. It is an important water hygiene indicator. High value implies that the water is seriously contaminated with sewage, organic matter, nitrate and phosphate. Water samples in the study showed considerable high values of BOD5. The values ranged from 5.720 mg/L to 7.170 mg/L. BH1 had the lowest BOD5 value while BH12 had the highest value. Generally, BOD5 values were higher than the acceptable value of 1 to 5 mg/L.
Bacteriological parameter
Bacterial analysis of the BHs water in the study area indicates the presence of fecal coliform. Fecal coliform is an indicator of bacterial contamination from excreta of human and other warm blooded animal. (Enetimi Idah et al. 2020). High level of fecal coliform in water can affect public health and community economy. Fecal coliform cause diseases like cholera, dysentery and diarrhea. The mean value of fecal and total coliform was 126 ± 31.40 MPN/100 mL and 287 ± 40.37 MPN/100 mL respectively. see Table 2. These values were above the acceptable limits set by NSDWQ which is 10MPN/100 mL. The possible source of these high coliform is from excreta of human or warm blooded animal diffusing into the BH form poorly constructed septic tank.
Heavy metals
The heavy metals like Zinc, Iron, Lead, Cadmium, Chromium and Manganese in the BH water were analyzed in Table 2. Some heavy metals have the potential of being carcinogenic, causing kidney failure, neurological disorder and metabolic dysfunction. According to NSDWQ, Zinc has no known health impact in drinking water. Measured mean value for Zinc in the study area was 0.06 ± 0.06 mg/L. The permissible limit for zinc in portable water is 3 mg/L. High concentration of Iron affect the colour and taste of water. In the study area, the mean value for Iron was 0.27 ± 0.06 mg/L. The permissible limit for Iron is 0.3 mg/L. Lead is implicated in serious health issues like cancer, interference with vitamin D metabolism, toxic to central, peripheral nervous system and obstruction of proper infant mental development (Saheed and Abimbola 2021), measured mean value for Lead was 0.0137 ± 0.005 mg/L, BH6 and BH7 had measured values 0.002 mg/L and 0.003 mg/L respectively, others had values above permissible limits of 0.01 mg/L. Cadmium is a heavy metal known for its toxicity to kidney (Abdullahi et al. 2020)., mean measured value of Cadmium in the BHs was 0.002 ± 0.001 mg/L, this value was lower than the acceptable limit of 0.003 mg/L set by NSDWQ. Chromium is known to be carcinogenic especially Cr+6, mean measured range of chromium in the study area was 0.010 mg/L to 0.090 mg/L, BH3, BH13, BH14 and BH15 had Chromium values within acceptable limit, others had values above acceptable limits of 0.02 mg/L.
Water quality index
Calculated results of WQI for BH water are presented in Table 5. BH7 had WQI in the range of (26–50), which is regarded as very good. BH1, BH3, BH4, BH8, BH11, BH14 and BH16 had range (51 to 75), water quality in this range is regarded as poor. BH2, BH5, BH6, BH9, BH12, BH13 and BH15 had range (76 to 100), water quality in this range is regarded as very poor. BH10 had WQI in range > 100; hence is not portable.
Correlation analysis of water parameters
Correlation coefficient was used to determine the interrelation and common source of the investigated parameters Table 6. Significant correlation coefficient (r) were taken at a = 0.05* and a = 0.01**. Strong correlation (r = 0.999) were seen in salinity and EC, salinity and TDS, BOD5 and nitrates, BOD5 including total coliform at (a = 0.05); (r = 0.989) in TDS and conductivity at (a = 0.05); (r = 0.837) in pH and total hardness at (a = 0.05); (r = 0.862) in alkalinity and calcium, alkalinity and magnesium at (a = 0.05); (r = 0.509) TH and calcium, TH and magnesium at (a = 0.01). Negative correlation (r = 0.508) were found in DO and BOD5, DO and nitrates, DO and total and fecal coliform at (a = 0.01). From the above correlation, it can be deduced that salinity observed is due to the presence of soluble minerals from rocks. The EC is due to dissolved cations and anions from rock bearing minerals in the ground. High pH is due to the presence of soluble magnesium and calcium carbonates. Nitrate is one of the products of decomposition of organic waste. Availability of nitrates promotes the proliferation of bacteria. High value of Total and fecal coliform increases the consumption of oxygen which results in low DO and high BOD5. Therefore, high values of nitrate, BOD5, and coliform are direct indicators of poor quality of water.
Principal component analysis
Principal component analysis (PCA) was applied to sets of parameters obtained from BHs water from the study area. PCs were extracted with their corresponding component plot rotated in space as shown in Tables 7 and 8 and Fig. 2. PC1 of 23.49% has a factor loading on BOD5, nitrates, total coliform and fecal coliform. This indicates presence of organic waste which could be from sewage leakage or prolong use of fertilizer for agricultural purpose. Therefore, the source of pollutant is mainly anthropogenic. PC2 of 19.77% has a factor loading on salinity, conductivity, TDS, turbidity, total hardness. The source of these pollutants is mainly from leaching rock bearing minerals from the ground, hence natural source. PC3 explains 13.98% of the total variance with factor loading on pH, total hardness, magnesium, calcium and lead. The possible reason for total hardness is the presence of calcium and magnesium ion. This account for the low acidity observed on the BHs water. Lead appears to come from anthropogenic source. PC4 explains 11.26% of the total variance characterized by factor loading on temperature, alkalinity and calcium. PC5 and PC6 explain 10.72% and 8.72% respectively with factor loading on manganese and cadmium respectively. These are the only heavy metals that appear as pollutant in this study, the possible source is anthropogenic.
Cluster analysis
The CA result of sixteen BHs in Okobo is presented as a Dendrogram as shown in Fig. 3. BHs were grouped into three clusters. Cluster 1 has five BHs; BH7, BH10, BH8, BH11, and BH12, BHs in this cluster tend to have the highest total and fecal coliform, nitrates, BOD5, turbidity, EC, lowest DO and heavy metals concentration. Cluster 2 has seven BHs; BH4, BH5, BH6, BH9, BH15 and BH16, this cluster tends to have the highest pH, alkalinity, Calcium, Magnesium, salinity, TDS, Iron and Zinc concentration. Cluster 3 has four BHs; BH1, BH2, BH3 and BH13, this cluster is characterized with BHs with lowest fecal and total coliform, nitrates, BOD5, conductivity, salinity, Calcium, Magnesium, Cadmium and Manganese, however, highest DO, pH, temperature and Chromium.
Conclusion
Physicochemical, bacteriological and heavy metal analysis of BHs water in Okobo, Akwa Ibom state shows that the water is slightly acidic, most physicochemical parameters measured were within the acceptable limits set by NSDWQ. However, DO, BOD5, nitrate, fecal and total coliform, lead, chromium and manganese were not within the limit set by NSDWQ. PCA shows that there are six principal pollutants whose sources are mostly organic waste from human excreta probably due to proximity of BH with broken or bad casing to septic tank and natural source due to leaching of minerals from rocks. CA shows that there are three groups of BHs with these pollutants. Water quality index calculation shows that only BH7 was portable, others require treatment. Since the major pollutant is fecal coliform, there is need to site BHs 15 m away from septic tank, BH casing should be constructed during the construction phase, calculated amount of high test hypochlorite (HTH) should be used to treat the water before consumption, Individuals and BHs owners should be adequately educated on the health impact associated with sitting BHs close to pollution source (WHO 2007). Finally, regular monitoring and evaluation system should be put in place to periodically monitor the suitability and pollution state of ground and surface water.
References
Abdullahi AA, Ighalo JO, Ajala OJ, Ayika S (2020) Physicochemical analysis and heavy metals remediation of pharmaceutical industry effluent using bentonite clay modified by H2SO4 and HCl. J Turkish Chem Soc Sect A 7:727–744
Adetinuke A, Oshunrinade O (2016) Comparison of water quality from boreholes and hand dug wells around and within Lagos, Nigeria. Int J Res Environ Stud 93(3):100
Ajala OJ, Ighalo JO, Adeniyi AG, Samuel O, Comfort AA (2020) Contamination issues in sachet and bottled water in Nigeria: a mini-review. Sustain Water Resour Manage 6:112. https://doi.org/10.1007/s40899-020-00478-5
Amadi AN (2011) Assessing the effect of Aladimma dumpsite on soil and ground water using water quality index and factor analysis. Aust J Basic Appl Sci 5(11):763–770
American Society for testing and materials (ASTM) (2016). International standard methods for acidity and alkalinity of water, ASTM D1067-16. Conshohocken, Philadelphia, USA. https://doi.org/10.1520/d1067-16
Asibor G, Ofuya O (2019) Well water quality assessment using water quality index in Warri metropolis, Delta State, Nigeria. Int J Environ Pollut Res 7(3):45–52
Chatterrji C, Raziuddin M (2002) Determination of water quality index of a degraded river in Asanol industrial area, Raniganj, Burdwan west Bengal. Nat Environ Pollut Technol 1(2):181–189
David AC, Jose PA, Rafaela SF (2020) Water quality assessment based on multivariate statistics and water quality index of a strategic river in the Brazil Atlantic Forest. Forest Sci Rep 10:22038. https://doi.org/10.1038/s41598-02078563-0
Elemile OO, Raphael DO, Omole DO (2019) Assessment of the impact of abattoir effluent on the quality of groundwater in a residential area of Omu-Aran. Nigeria Environ Sci Europe 31:16. https://doi.org/10.1186/s12302-019-0201-5
Enetimi IS, Felix OY, Sylvester CI (2020) Bacteriological quality of groundwater in Imiringi town, Bayelsa State, Nigeria. J Biotechnol Biomed Sci 2(2):34–40. https://doi.org/10.14302/1ssn.2576-6694.jbbs-20-3340
Enyoh CE, Andrew WV, Ngozi JE (2018) pH variation and chemometric assessment of borehole water in Orji. Owerri imo state, Nigeria. J Environ Anal Chem 5:2. https://doi.org/10.4172/2380-2391.1000238
Essumang DK, Senu J, Fianko J, Nyarko B, Adokoh C, Boamponsem L (2011) Groundwater quality assessment: a physicochemical properties of drinking water in a rural setting of developing countries. Can J Sci Indus Res 2(3):102–126
Ighalo J, Adeniyi AG (2020) A comprehensive review of water quality monitoring and assessment in Nigeria. https://doi.org/10.1016/j.Chemosphere.2020.127569
Ighalo JO, Adewale GA, Jamiu A, Samuel O (2021) A systematic literature analysis of the nature and regional distribution of water pollution sources in Nigeria. J Cleaner Prod 283:124566. https://doi.org/10.1016/j.jclepro.2020.124566
Kaiser HF (1985) The Verimax criteria for analytical rotation in factor analysis. Psychometrika 23(3):187–200
Marcovecchio JE, Botte SE, Freije R (2007) Heavy metals, major metals, trace elements. CRC Press, London, Handbook of water analysis. Second edition, pp 275–311
Mohammed AD, Olasehinde PI, Amadi AN, Christopher U (2016) Evaluation of ground water in parts of Abuja, Nigeria using factor analysis and water quality index. In: Proceedings of the international conference on Education, development and innovation (INCEDI) Ghana
Paschke SS, Harrison WJ, Walton-Day K (2016) Effects of acidic recharge on groundwater at the St. Kelvin Gulch site, Leadville, Colorado. Geochem Explor Environ Anal 1(1):3–14
Saheed AG, Abimbola TO, Azeem AA (2021) Assessment of heavy metals contamination and associated risk in shallow groundwater sources from three different residential areas with Ibadan metropolis, south Nigeria. Appl Water Sci 11:81. https://doi.org/10.1007/s13201-021-01414-4
Standard organization of Nigerian, SON (2015) Nigerian standard for drinking water quality. NIS 554-2015
Udoh A, Lawal BK, Akpan M, Labaran KS, Ndem E, Ohabunwa U (2021) Microbial contamination of packaged drinking water in Nigeria. Trop Int Health 26:1378–1400. https://doi.org/10.1111/tmi.13672
Ugochukwu E, Nnaemeka OA, Stephen UN (2021) An appraisal of data collection, analysis and reporting adopted for water quality assessment: a case of Nigerian water quality research. Heliyon. https://doi.org/10.1016/j.heliyon.2021.e07950
United states environmental protection agency (USEPA) (2002) Total coliform and Escherichia coli in water by membrane filtration using a simultaneous detection technique (MI medium). USEPA method 1604, Washington D.C, USA, pp 1–14
WHO (2004) Guidelines for drinking water, vol. 1, Recommendations (3rd edition). Geneva, Switzerland. http://whqlibdoc.who.int/publications/2004/9241546387. Accessed 29 July 2013
WHO (2007) Water for pharmaceutical use. Quality assurance of pharmaceuticals: Second updated edition, vol 2. World Health Organization, Geneva pp 170–187
Wu TN, Huang YC, Lee MS, Kao CM (2005) Source identification of groundwater pollution with the aid of multivariate statistical analysis. Water Sci Technol Water Supply 5(6):281–288
Funding
The author declare that there was no funding received from any private or public organisation.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there is no conflict of interest in the submitted work.
Human and animal rights
The authors declear that there was no violation of human or animal right during the collection of samples for this work. No human being or animals specimen were used in this work.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Umana, I.M., Neji, P.A. & Agwupuye, J.A. Assessment of underground water quality in Okobo local government area of Akwa Ibom State, Nigeria. Appl Water Sci 12, 106 (2022). https://doi.org/10.1007/s13201-022-01614-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13201-022-01614-6