Abstract
The present research studied the antibacterial effect of silver-coated Ni0.5Zn0.5Fe2O4 magnetic nanoparticles on Gram-negative bacteria Escherichia coli (E. coli) from water. The effects of pH (6, 7 and 9), disinfectant dose (2, 5 and 10 g/L) and contact time (10, 20 and 30 min) have been also investigated. To obtain important factors, the interactions between factors and optimal experimental design in surface response method were used based on Box–Behnken design. According to the research findings, the system is efficient in eliminating E. coli. The results showed that E. coli elimination efficiency intensified through increasing the amount of nanoparticles from 2 to 10 g/L. The results also demonstrated no significant change in E. coli elimination through pH increasing of 6 to 9. Expanding contact time from 10 to 30 min also heightened E. coli elimination rate. R2 for E. coli elimination is 0.9994 indicating a good agreement between model experimental data and forecasting data.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Different countries throughout the world are concerned for supplying drinking water requirements due to population growth and depletion of drinking water sources. Water scarcity seriously resulted from increasing environmental pollution has turned water supply and sanitary requirements into one of the main issues of the present world (Thatai et al. 2019). Diseases caused by water contamination have led to the death of tens of thousands people around the world. However, the possibility of refining water provides access to resources for several purposes, and may, in some cases, compensate for water resources scarcity (Plessis 2019). Increasing the production and consumption in various industries, natural and artificial pollutants have challenged access to traditional water treatment practices to achieve the standard as the pollutants approaching to surface and groundwater resources. Sewage discharge to water resources is of water pollution sources to be identified, controlled and deterred. The first step is to monitor water pollution. Particular organisms including coliform family must be applied rather than monitoring all parameters to assess pathogenic microbial contamination. Coliform family is not pathogenic and remains in the environment for a relatively long time in large numbers; further, as they are exclusively intestinal, their presence in the environment is an indication of fecal contamination (Ashbolt 2004). Coinciding with the start of drinking water disinfection with chlorine in 1904, outbreak of epidemics associated with contaminated water consumption was severely reduced (Wolf et al. 2018). Then, different methods of drinking water disinfection like ozone and UV light for infection prevention were applied. However, because of numerous advantages of chlorine and its derivatives, drinking water disinfection using these compounds is the most global common method of disinfection (Alicia and Alvarez 2000; El-Shafy and Grünwald 2000). Using new methods is inevitable on account of population growth, the need for water supply and sanitary requirements due to widespread pollution of water sources. Today, nanotechnology is proposed to solve the issues of water quality and quantity (Vikesland 2018). The effect of metal ions on water disinfection has been studied by many scientists (Jain and Pradeep 2005; Deng et al. 2017; Fan et al. 2018; Mnatsakanyan and Trchounian 2018; Motshekga et al. 2018; Park et al. 2018). Silver, copper and zinc ions have been long known for their antimicrobial properties. Some studies have shown that the metal ions react with proteins through binding to sulfhydryl groups (–SH) in enzymes, and finally disable proteins (Yoon et al. 2007). If the metals are tiny, they would show better antimicrobial properties as a result of increased surface to volume ratio (Zhang et al. 2008). The metal nanoparticles can be used for coating some parts for antimicrobial properties and filters in medical equipment. Using the materials endures some challenges including microbial resistance against chemical antimicrobial agents as well as producing disinfection using conventional disinfectants. Innovation in new technologies development of disinfection and water treatment is a new achievement in the field of nanotechnology. Escherichia coli bacteria are of a streptococcus genus, Gram-positive organism, catalase-negative, oval and non-sporulation, facultative (lactic acid production from lactose) with complex nutritional requirements that often exist in most vegetables, herbs and foods, especially foods of animal origin such as dairy products. Also, it is part of normal intestinal flora of some mammals and humans (Rezaei-Zarchi et al. 2010). Berendjchi et al. (2011) performed a study to evaluate the activity of copper nanoparticles prepared in the form of a coating on cotton layer through sol–gel method. The results revealed that nanoparticles are effective in both E. coli (Gram −) and S. aureus (Gram +) (Berendjchi et al. 2011). Dong et al. (2011) analyzed antibacterial activity of magnetic nanoparticles (Fe3O4). According to the research results, modified nanoparticle is more effective against E. coli bacteria. Nanoparticles in a magnetic field can be recovered from sample (Dong et al. 2011). Cortés et al. (2006) conducted a study to investigate magnetic properties and antibacterial activity of a quad-core copper complex. The results displayed that the 4-phenylamidazole complex is effective for cereus bacteria and other Gram-positive bacteria, whereas complex pyridine N-oxide and 2-methylamidazole are only effective against Gram-negative bacteria (Cortés et al. 2006). Sanpo et al. (2013) examined spinel ferrite nanoparticle antibacterial activity using citric acid as a chelating agent through sol–gel method. According to the research findings, zinc and copper substitution in nano-cobalt ferrite particles significantly increases E. coli and Staphylococcus aureus antibacterial activity (Sanpo et al. 2013). Tian et al. (2014) explored a nanocomposite antibacterial activity consisting of iron oxide, silver oxide and graphene oxide nanoparticles as the core, and only compared silver nanoparticles antibacterial activity. According to the research findings, obtained nanocomposites (GO-IONP-Ag) showed more powerful antimicrobial activity than silver nanoparticles; moreover, antibacterial effect was also observed on Gram-negative bacteria (E. coli) and Gram-positive bacteria (S. aureus) (Tian et al. 2014). Nickel–zinc ferrites have drawn noticeable consideration from researchers because of their remarkable magnetic properties, large permeability and very high electrical resistivity (Sharma et al. 2010). They have an extensive list of potential applications in such areas as high–density information storage devices, microwave devices, transformer cores, magnetic fluids (Liu et al. 2018), etc. We thus hypothesize that silver-coated Ni0.5Zn0.5Fe2O4 magnetic nanoparticles are viable filtration–sorption media to consider for practical application of bacteria removal. These nanocomposites are considered as one of the most suitable alternatives for magnetic and biochemical applications, considering the economic aspects and the properties of biodegradability and non-toxicity. Silver-coated Ni0.5Zn0.5Fe2O4 magnetic nanoparticles can have a very favorable outlook for the water and wastewater industry, taking into account advantages such as desirable health, economic and environmental aspects, as well as high efficiencies in the removal of microbial contaminants from water and wastewater, which may lower human dependence on chlorine. The present study aimed to evaluate the effect of silver-coated red soil on E. coli removal as a water microbial pollution index and effects of some parameters on its efficiency.
Materials and methods
General
This is an applied research carried out at a laboratory experimenting lyophilized strains of E. coli: ATCC 25922 prepared by a center of fungi collection and industrial bacteria in Iran. The materials used in this study include zinc chloride, nickel chloride, iron chloride, hydrochloric acid, sodium chloride, silver nitrate, sodium borohydride and culture media of lauryl sulfate broth, brilliant green bile broth, EC broth, eosin methylene blue, azide dextrose broth, bile esculin azide agar, Brain heart infusion, R2A agar, nutrient broth and triple soy broth produced by Merck and Chem Lab company. B150 Nabertherm model furnace, SSIMS ONOS SW3H ultrasonic bath machine, Shaker incubator machine 8480 and also Rigaku RAD-IIA spectrometer with radiation of (1.5418 Å), 40 kV and 30 mA were also applied. The experiments were carried out according to the standard methods contained in the standard methods for water and sewage testing (Apha 2012). The magnetic characteristic was investigated by vibrating sample magnetometer (VSM, LDJ9600).
Synthesis of Ni0.5Zn0.5Fe2O4
Sixty ml of egg white was poured into a container and diluted with 100 ml distilled water; next, it was poured into a beaker for uniformity, then placed on a heater stirrer so that a homogeneous mixture is obtained. In the next step, 1.48, 1.45 and 8 g FeCl3, NiCl2 and ZnCl2 salts were weighed and added to a small amount of distilled water, then mixed and finally added to the homogeneous egg whites. The mixture was quickly stirred for 30 min. Following, it was heated at 80 °C for drying and turning into the powder. The obtained powder at this stage was then placed in the furnace at 500 °C for 2 h, and magnetic nanoparticles were prepared (Gabal et al. 2012).
Synthesis of Ni0.5Zn0.5Fe2O4/Ag nanocomposite
In this procedure, 3 g of Ni0.5Zn0.5Fe2O4 was dispersed in 30 mL of sodium borohydride (NaBH4) solution with constant stirring. To this mixture, 60 mL of a 0.1 M silver nitrate solution was added drop by drop. After all the silver nitrate solution is added, the mixture was further stirred for 30 min. The prepared Ni0.5Zn0.5Fe2O4/Ag nanocomposite was then separated from solution by magnetic decantation and washed three times with distilled water, dried at 60 °C overnight and milled to achieve smooth powder. Finally, it was stored in a dark container. The inductively coupled plasma atomic emission spectroscopy (ICP-AES) was applied for determining the amount of the Ag nanoparticle loading on magnetic nanoparticles. After coating silver nanoparticles on Ni0.5Zn0.5Fe2O4, its effect on disinfection and removal of Gram-negative bacterium of E. coli was investigated.
Culture medium
Escherichia coli species were cultured according to the manufacturer guidelines. Briefly, a single colony of E. coli was taken from a refrigerated stock and pre-cultured in 20 mL tryptic soy broth (TSB) by incubation at 37 °C for 24 h. Then, it was transferred into tryptic soy agar (TSA) and incubated for 24 h at 37 °C. The top of each colony was touched with a sterile loop, and the growth was transferred into a tube containing 4 to 5 mL of distilled water (IROST 2016). A McFarland standard 0.5 was used to determine the cell concentrations. The cell density was compared to that standard using a UV/VIS spectrophotometer, an equivalent optical density of 0.1 at 625 nm with regard to the calibrated standard cell suspensions in distilled water (Dhara and Tripathi 2013). A barium sulfate turbidity standard was used to standardize the inoculums density for a susceptibility test; its turbidity was equivalent to that of a 0.5 McFarland standard the latter made according to Garcia (2010). To obtain the required cell suspensions, the stock was serially diluted in distilled water. This resulted in a suspension containing approximately 102, 104 and 106 CFU/mL. The standard plating method was applied to confirm the bacterial concentrations. This test was done in triplicates on tryptic soy agar. Samples were plated in triplicates. The colonies were visually identified and counted after incubation at 37 °C overnight.
Response level method
The response level method is a collection of useful statistical and mathematical methods for modeling and analyzing problems, in which the desired response level is affected by multiple variables (Ahmadi et al. 2005). In recent years, the method has been well considered in the field of water treatment, as it is very easy with a quick and accurate design. The method provides a second-order polynomial model to fit the test responses as follows (Myers et al. 2004):
where Y is the response, (X1, X2 and X3) are the encoded variable factors, b0, bi and bij (i, j = 1, 2, 3) are the model estimated coefficients.
In the response level method, the Box–Behnken design (BBD) has been used to optimize the responses. The parameters significance level was 95%. To eliminate E. coli, from contaminated water, the effect of nanoparticle factors, pH and contact time on removing E. coli has been investigated. The factors’ levels are presented in Table 1. Regarding the number and levels of selected factors using response level and Box–Behnken design method, through Design-Expert software 8.0.1, 15 tests have been introduced in the proposed range. The results were analyzed using ANOVA table and 3D charts.
To calculate the removed amount of E. coli by nanoparticle, the following equation is used:
where R represents the percentage of bacterial elimination, C0 is the number of primary bacteria, and Ct is the number of bacteria remaining after disinfection time t.
Results and discussion
Characterization of Ni0.5Zn0.5Fe2O4/Ag magnetic nanoparticles
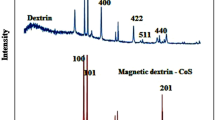
Ni0.5Zn0.5Fe2O4 magnetic nanoparticles were synthesized according to the reported procedure by Gabal et al. (2012) and fully characterized based on our previous reports (Beigzadeh and Moeinpour 2016; Omidvar-Hosseini and Moeinpour 2016). The inductively coupled plasma atomic emission spectroscopy (ICP-AES) was applied for determining the amount of the Ag nanoparticle loading on Ni0.5Zn0.5Fe2O4. It showed 0.071 mol of silver nanoparticle per gram of the prepared disinfectant.
In order to verify the formation of silver layer on Ni0.5Zn0.5Fe2O4 magnetic nanoparticles, UV spectra were taken from both samples, as shown in Fig. 1. The peak formed in a wavelength of about 350 nm in the UV spectrum of silver nanoparticles (b) reveals the presence of a silver layer on magnetic nanoparticles (Wang et al. 2011).
The magnetic characteristics of the Ni0.5Zn0.5Fe2O4/Ag magnetic nanoparticles were identified using vibrating sample magnetometer (VSM). As can be seen in Fig. 2, M(H) hysteresis loop was completely reversible for the sample, indicating that the Ni0.5Zn0.5Fe2O4/Ag magnetic nanoparticles show super paramagnetic characteristics. The hysteresis loops of them reached saturation up to the ultimate applied magnetic field. At room temperature, the magnetic saturation value of the Ni0.5Zn0.5Fe2O4/Ag magnetic nanoparticles is about 25 emu g−1. The high permeability of particles in the magnetization showed that they could be separated by a typical magnetometer.
Development and evolution of prediction model
Table 2 shows experimental results of eliminating E. coli according to the design table with the response level and BBD with Ni0.5Zn0.5Fe2O4 magnetic nanoparticles.
The results of analysis of variance (ANOVA) for the independent variables are presented in Table 3. The confidence level was considered 95%. For this reason, P ratio must be less than 0.05 so that the model or the factors effect is significant. This means that there is a 5% probability of error that a non-important factor is considered important. One of the most important factors in the statistical analysis of F ratio is the model statistical significance. If the F ratio of an agent is higher for, it indicates that this factor is significant and its effect on the response rate is more important. The analysis of variance for the removal of E. coli is shown in Table 3.
Considering P value and F ratios of eliminating E. coli, the effect of pH factors, disinfectant dosage and contact time on E. coli elimination is significant. Disinfectant dosage factor has the greatest effect on E. coli removal. Other factors showed no significant relationships.
Mathematical model
The value of R2 for E. coli elimination is 0.9994 indicating a good agreement between experimental data and the model predicted data. The comparison of the experimental model and predicted results of E. coli removal rate is presented in Fig. 3.
Also, insignificance of the term not fitted in Table 3 implies that the proposed statistical model provided is well fitted to the experimental data. The second-order statistical model, offering the design of E. coli removal rate in terms of agents actual values (not coded values), is presented as follows:
where disinfectant dosages (A) are in g/L, contact time (C) in minutes, and elimination of E. coli (R1) is in percentage.
The effect of parameters
The effect of Ni0.5Zn0.5Fe2O4/Ag magnetic nanoparticles dosage
The Ni0.5Zn0.5Fe2O4/Ag magnetic nanoparticles dosage is one of the most important factors, as shown in Fig. 4a, b (3D diagrams); bacteria elimination rate has been raised by intensifying nanoparticles dosage in the process of bacterial elimination, for example for an increased amount from 2 to 5 g for E. coli, the elimination percentage has increased from 95.7 to 100%, which can be attributed to an escalation in the contact surface of the nanoparticles with bacteria; as well as an increase in hydrogen peroxide concentration produced from nanoparticles intensifying bacteria elimination percentage (Sawai et al. 1996). The results of the present study are consistent with Zhang et al. (2007). It should be noted that in these experiments, the control test was performed at following conditions:
Contact time = 20 min.; disinfectant concentration (uncoated Ni0.5Zn0.5Fe2O4) = 0.05 g/mL; E. coli no. = 104 CFU/mL. After culture on the medium (R2A agar), the bacteria grew and formed a colony. The bacterial inactivation percent was zero.
The effect of contact time
Contact time is the second most important factor. As shown in Fig. 4a, b for E. coli, the increase in contact time has led to an increase in bacterial elimination percentage. For example, an increase from 10 to 20 min heightened E. coli removal percentage from 95.1 to 100%, which results in expanded process time.
The effect of pH
The effect of pH factor on removing understudied bacteria has been lower than other investigated factors. In spite of the removal research in the field of chemistry, it is associated with living organisms that the organisms’ reactions may cause pH changes. As shown in Fig. 4a, b (diagrams of E. coli removal), the results indicated no significant change by a pH change of 6 to 9 in E. coli elimination percent. It is interpreted that the intestinal bacteria are not pH-tolerant. The results of the study are consistent with Alikhani et al. (2012) and Haavik (1974). The control test was performed at the conditions described above.
Optimization
Respecting economic dimension, the software has the optimum point with a 97.2% elimination rate for E. coli and a concentration of 3.2 g/L magnetic nanoparticles, a pH of 6.35 and a contact time of 15.3 min. Moreover, if the optimal point is determined by economic dimension, the maximum removal at these points equals 79.6%, at a point where the concentration of nanoparticles = 2 g/L, pH = 6.16 and contact time = 11.4 min. If maximum removal percentage is the objective, regardless of economic dimension, the highest percentage of removal is 100% where nanoparticle concentration is 10 g/L, pH = 6.19, and contact time is 30 min.
The effect of the number of bacteria
Figure 5 illustrates that bacterial removal rate decreases by augmenting the number of bacteria. For instance, in an E. coli sample with an increase of 103 to 105 CFU/mL in the number of bacteria, disinfection efficiency decreases from 100 to 99.92% as nanoparticles contact surface with bacteria is declined by increased number of bacteria, and the turbidity is intensified, due to diminishing nanoparticles’ disinfection properties. The control test was performed at the conditions described above.
According to our results, the mechanism of bacterial inactivation by red soil coated Ag is ionic. The mechanism operates based on the transformation of microorganisms by converting—SH bonds to—SAg bonds. Silver nanoparticles disable the enzyme by releasing Ag+ ions and absorbing the—SH bonds which are the basis of the protein enzymes at the surface of bacteria. Therefore due to the lack of absorption of phosphate by the cell, the bacteria are inactivated. This mechanism does not terminate with the destruction of the bacteria and is a permanent process (Davies and Etris 1997).
Conclusion
The results of the present study demonstrated that Gram-negative E. coli bacterium is sensitive to Ni0.5Zn0.5Fe2O4/Ag magnetic nanoparticles. Intensifying nanoparticles concentration increases bacterial removal percentage; furthermore, raising the number of bacteria reduces nanoparticles disinfection properties. Extended contact time of the bacteria with magnetic nanoparticles may increase bacterial elimination percentage. The results also revealed no significant changes in E. coli elimination by increasing pH from 6 to 9. R2 value for E. coli elimination is 0.9994, implying a good agreement between the experimental model and predicted data. Of major advantages of disinfection is separating nanoparticles from solution by a magnetic force, which not only reuses disinfection and is economically feasible, but also prevents the entrance of nanoparticles into the environment. Scientific nano-advances, it is possible to use Ni0.5Zn0.5Fe2O4/Ag magnetic nanoparticles in water and wastewater industry. Respecting silver nanoparticles, one of the limitations considered as a disadvantage of using disinfectant is the high price. However, silver reusing and recycling may, to some extent, moderate the constraint of this strong disinfectant.
References
Ahmadi M, Vahabzadeh F, Bonakdarpour B, Mofarrah E, Mehranian M (2005) Application of the central composite design and response surface methodology to the advanced treatment of olive oil processing wastewater using Fenton’s peroxidation. J Hazard Mater 123:187–195
Alicia C, Alvarez J (2000) DBP formation during chlorination. J Am Water Works Assoc 92:76–90
Alikhani MY, Lee SM, Yang JK, Shirzad-Siboni M, Peeri-Dogaheh H, Khorasani MS, Nooshak MA, Samarghandi MR (2012) Photocatalytic removal of Escherichia coli from aquatic solutions using synthesized ZnO nanoparticles: a kinetic study. Water Sci Technol 67:557–563
Apha A (2012) Standard methods for the examination of water and wastewater, 22nd edn. WEF
Ashbolt NJ (2004) Microbial contamination of drinking water and disease outcomes in developing regions. Toxicology 198:229–238
Beigzadeh P, Moeinpour F (2016) Fast and efficient removal of silver (I) from aqueous solutions using aloe vera shell ash supported Ni0.5Zn0.5Fe2O4 magnetic nanoparticles. Trans Nonferrous Met Soc China 26:2238–2246
Berendjchi A, Khajavi R, Yazdanshenas ME (2011) Fabrication of superhydrophobic and antibacterial surface on cotton fabric by doped silica-based sols with nanoparticles of copper. Nanoscale Res Lett 6:594
Cortés P, Atria AM, Contreras M, Garland MT, Peña O, Corsini G (2006) Magnetic properties and antibacterial activity of tetranuclear copper complexes bridged by oxo group. J Chil Chem Soc 51:957–960
Davies RL, Etris SF (1997) The development and functions of silver in water purification and disease control. Catal Today 36:107–119
Deng CH, Gong JL, Zhang P, Zeng GM, Song B, Liu HY (2017) Preparation of melamine sponge decorated with silver nanoparticles-modified graphene for water disinfection. J Colloid Interface Sci 488:26–38
Dhara L, Tripathi A (2013) Antimicrobial activity of eugenol and cinnamaldehyde against extended spectrum beta lactamase producing enterobacteriaceae by in vitro and molecular docking analysis. Eur J Integr Med 5:527–536
Dong H, Huang J, Koepsel RR, Ye P, Russell AJ, Matyjaszewski K (2011) Recyclable antibacterial magnetic nanoparticles grafted with quaternized poly (2-(dimethylamino) ethyl methacrylate) brushes. Biomacromolecules 12:1305–1311
El-Shafy MA, Grünwald A (2000) THM formation in water supply in South Bohemia, Czech Republic. Water Res 34:3453–3459
Fan M, Gong L, Huang Y, Wang D, Gong Z (2018) Facile preparation of silver nanoparticle decorated chitosan cryogels for point-of-use water disinfection. Sci Total Environ 613:1317–1323
Gabal M, El-Shishtawy RM, Al Angari Y (2012) Structural and magnetic properties of nano-crystalline Ni–Zn ferrites synthesized using egg-white precursor. J Magn Magn Mat 324:2258–2264
Garcia L (2010) Preparation of routine media and reagents used in antimicrobial susceptibility testing. ASM, Washington
Haavik H (1974) Studies on the formation of bacitracin by Bacillus licheniformis: role of catabolite repression and organic acids. Microbiology 84:321–326
IROST (2016) Persian type culture collection 2016. Iranian Research Organization for Science and Technology. http://ptcc.irost.org/. Accessed 28 Jan 2019
Jain P, Pradeep T (2005) Potential of silver nanoparticle-coated polyurethane foam as an antibacterial water filter. Biotechnol Bioeng 90:59–63
Liu R, Yu Q, Liu Y, Liu X, Wang F, Xu Y (2018) Optimization of citrate-gel preparation process for magnetic Ni–Zn ferrite nanoparticles. J Nanosci Nanotechnol 18:2838–2843
Mnatsakanyan N, Trchounian A (2018) Nanocomposite filter made from porous mineral tuff with absorbed silver nanoparticles and its application for disinfection of water. J Water Supply Res Technol 67:127–136
Motshekga SC, Ray SS, Maity A (2018) Synthesis and characterization of alginate beads encapsulated zinc oxide nanoparticles for bacteria disinfection in water. J Colloid Interface Sci 512:686–692
Myers RH, Montgomery DC, Vining GG, Borror CM, Kowalski SM (2004) Response surface methodology: a retrospective and literature survey. J Qual Technol 36:53
Omidvar-Hosseini F, Moeinpour F (2016) Removal of Pb(II) from aqueous solutions using Acacia nilotica seed shell ash supported Ni0.5Zn0.5Fe2O4 magnetic nanoparticles. J Water Reuse Desalt 6:562–573
Park S, Ko YS, Jung H, Lee C, Woo K, Ko G (2018) Disinfection of waterborne viruses using silver nanoparticle-decorated silica hybrid composites in water environments. Sci Total Environ 625:477–485
du Plessis A (ed) (2019) Current and future water scarcity and stress. In: Water as an inescapable risk. Springer Water. Springer, Cham. https://doi.org/10.1007/978-3-030-03186-2_2
Rezaei-Zarchi S, Javed A, Javeed Ghani M, Soufian S, Barzegari Firouzabadi F, Bayanduri Moghaddam A, Mirjalili SH (2010) Comparative study of antimicrobial activities of TiO2 and CdO nanoparticles against the pathogenic strain of Escherichia coli. Iran J Pathol 5:83–89
Sanpo N, Wen C, Berndt CC, Wang J (2013) Antibacterial properties of spinel ferrite nanoparticles. Microbial pathogens and strategies for combating them: science, technology and education. Formatex Research Centre, Spain, pp 239–250
Sawai J, Kawada E, Kanou F, Igarashi H, Hashimoto A, Kokugan T, Shimizu M (1996) Detection of active oxygen generated from ceramic powders having antibacterial activity. J Chem Eng Jpn 29:627–633
Sharma S, Verma K, Chaubey U, Singh V, Mehta B (2010) Influence of Zn substitution on structural, microstructural and dielectric properties of nanocrystalline nickel ferrites. Mater Sci Eng B 167:187–192
Thatai S, Verma S, Khurana P, Goel P, Kumar D (2019) Water quality standards, its pollution and treatment methods. In: Naushad M (ed) A new generation material graphene: applications in water technology. Springer, Cham. https://doi.org/10.1007/978-3-319-75484-0_2
Tian T, Shi X, Cheng L, Luo Y, Dong Z, Gong H, Xu L, Zhong Z, Peng R, Liu Z (2014) Graphene-based nanocomposite as an effective, multifunctional, and recyclable antibacterial agent. ACS Appl Mater Interface 6:8542–8548
Vikesland P (2018) Nanosensors for water quality monitoring. Nat Nanotechnol 13:651–660
Wang L, Luo J, Shan S, Crew E, Yin J, Zhong CJ, Wallek B, Wong SS (2011) Bacterial inactivation using silver-coated magnetic nanoparticles as functional antimicrobial agents. Anal Chem 83:8688–8695
Wolf J, Hunter PR, Freeman MC, Cumming O, Clasen T, Bartram J, Higgins JP, Johnston R, Medlicott K, Boisson S, Prüss-Ustün A (2018) Impact of drinking water, sanitation and handwashing with soap on childhood diarrhoeal disease: updated meta-analysis and meta-regression. Trop Med Int Health 23:508–525
Yoon KY, Byeon JH, Park JH, Hwang J (2007) Susceptibility constants of Escherichia coli and Bacillus subtilis to silver and copper nanoparticles. Sci Total Environ 373:572–575
Zhang L, Jiang Y, Ding Y, Povey M, York D (2007) Investigation into the antibacterial behaviour of suspensions of ZnO nanoparticles (ZnO nanofluids). J Nanopart Res 9:479–489
Zhang L, Ding Y, Povey M, York D (2008) ZnO nanofluids—a potential antibacterial agent. Prog Nat Sci 18:939–944
Acknowledgements
The authors acknowledge the Islamic Azad University-Bandar Abbas Branch for financial support of this study.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Asadi, S., Moeinpour, F. Inactivation of Escherichia coli in water by silver-coated Ni0.5Zn0.5Fe2O4 magnetic nanocomposite: a Box–Behnken design optimization. Appl Water Sci 9, 23 (2019). https://doi.org/10.1007/s13201-019-0901-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13201-019-0901-4