Abstract
The effects of small-scale gold mining activities on heavy metal levels in groundwater were determined for the Lower Pra Basin of Ghana. Sixty five boreholes in 45 communities were analysed between January 2012 and April 2012 for the dry season and June and October 2012 for the wet season. The test for significance and the use of cluster analysis, a multivariate approach, clearly delineate Cu, Zn, Cd and Hg as possible anthropogenic contributors, whilst As, Se and Pb could be both anthropogenic and natural geochemical process contributors of metal level concentrations in the groundwater. The analysis shows generally low pH values in the basin with more than 95% of dry season and almost all wet season values being acidic or slightly acidic. Approximately 35% of boreholes in the wet season recorded values for cadmium above WHO guideline value of 3.0 µg/l with almost all dry season values falling below 3.0 µg/l except for two boreholes. Generally, high values were recorded for iron, manganese, lead, Al and Hg. In general, lead, copper, cadmium and manganese showed higher wet season values than dry season suggesting anthropogenic influence.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Water is essential for the existence of man and all forms of life. However, this valued resource is increasingly being threatened as human populations increase and demand more water of high quality for domestic purposes and economic activities (Ackah et al. 2011). Even though Ghana is well endowed with water resources, its availability is decreasing owing to climate variability and change, rapid population growth, increase in environmental degradation, pollution of rivers and draining of wetlands (WRC 2008; Ackah et al. 2011). The preference of groundwater to surface water as a source of drinking water was based on the fact that groundwater excluded from the atmosphere would be less susceptible to pollution. This is true to an extent. However, groundwater in hard-rock aquifers, particularly in mining areas, such as the Pra Basin is known to be vulnerable to quality problems, because the rocks are often carbonate deficient and give rise to poorly buffered water that may have serious impact on human health (Smedley et al. 1995). Secondly, in gold and base metal mining areas, sulphide oxidation resulting from chemical and biogeochemical processes leads to the production of low pH groundwater that encourages the dissolution of trace metals into the groundwater system in very high concentrations. The groundwater, thus, becomes dangerous for human consumption (Kortatsi 2006).
Ghana has been known to be a gold producer since the fifteenth century and currently Africa’s second most important producer of gold after South Africa (Coakley 1999). The sector is said to have attracted over US $3 billion of foreign direct investment by 1999 and now accounts for over 30% of gross foreign earnings.
Artisanal gold mining is said to be on the increase in the Pra Basin, and it is said to be the major contributor of metals in surface and groundwater within the basin because of indiscriminate use of mercury (Hg) and other chemicals, which are detrimental to human health during mining activities (Donkor et al. 2006). Small-scale gold mining all over the world is noted for its effects on water bodies through pollution of both ground and surface waters, because its activities by nature make use of a lot of water thereby seriously polluting water resources (Cunningham et al. 2005; Owens et al. 2005).
Small-scale (artisanal) mining has been defined differently around the world. In Ghana, however, small-scale (gold) mining is defined as“…mining (gold) by any method not involving substantial expenditure by an individual or group of persons not exceeding nine in number or by a cooperative society made up of ten or more persons” (Gov. of Ghana 1989). Generally, it is a term used to describe gold mining activities that uses simple methods (e.g. pickaxes, chisels, sluices and pans) to extract and process gold on a small scale (Shoko and Love 2005). Two types of small-scale miners exist, legal and illegal. Legal small-scale gold miners refer to those who have acquired mining licences from the Minerals Commission of Ghana to cover their concessions. An area of 25 acres is the maximum allowable area that is allocated to each person or group of person. Illegal operators, on the other hand, work without licences, have no concessions of their own; their operations are furtive and clandestine in nature often initiating confrontations with both state law enforcement agencies and the security personnel of large-scale mining companies, operating uncontrollably within the concessions of large-scale mining companies or in areas prohibited for mining such as forest reserves and environmentally sensitive areas (Appiah 1998). Artisanal gold mining (AGM) is one such anthropogenic activity that has resulted in the use of an enormous amount of metallic mercury. The mercury used by the miners is usually discharged in an abusive manner into ecosystems (Pfeiffer and Larceda 1988; Meech et al. 1998). Elemental Hg, which is now known to spread very effectively from diverse sources to both terrestrial and aquatic systems, is rampantly used in the Lower Pra Basin. Small-scale gold miners invariably prefer free milling ores (not sulphidic ores), using sluicing, as the main method for processing by gravity concentration (Aryee et al. 2002).
The ore is first crushed into pebbles by physical or mechanical means. The pebbles undergo primary, secondary and tertiary grinding and are transferred to the riverside or pond in cloth bags to be refined by amalgamation (Amegbey and Eshun 2003).
Mercury is often poured on the milled concentrate in a pan and mixed by hand with the concentrate, forming a lump or ball of mercury–gold amalgam. Water is added several times to discard tailings and remove lighter particles until only the amalgam remains, which is then squeezed in a piece of cloth to recover excess mercury (often re-bottled and used again).
The basin is drained by numerous springs, streams and the major river, River Pra which originally served as potable water sources to most communities within the basin (Dickson and Benneh 1980). Owing to the upsurge in the mining activities, leading to the discharge of mine effluents into the river and other water bodies in the basin through small-scale mining activities, several of these surface water resources have become contaminated and therefore unsuitable for potable use. The Government of Ghana, with the help of donor countries increasingly apprehensive of the extent of pollution of surface water resources, has been providing communities with drilled borehole and hand dug wells as alternative source of potable water supply.
Natural geochemical and biochemical processes such as oxidation of pyrite and arsenopyrite in an aquifer which causes groundwater to be prone to chemical contamination do not only threaten the quality of human health but also pose a threat to sustainable development and management of groundwater resources (Kortatsi 2006).
The existence of sulphites in the rock matrix and the low pH groundwaters, as shown by Ahialey et al. (2010) and Dorleku (2013) within the Pra Basin, suggest that there could be a high mobilisation of toxic metals into the groundwater, and for that matter the drinking water system of the Basin. This paper, therefore, seeks to examine the level of heavy metal concentration within the Lower Pra Basin as a result of mining activities principally by small-scale miners.
Multivariate statistical technique such as cluster analysis (CA), discriminant analysis (DA) and principal component analysis (PCA) are environmetric methods deemed to be the best approach in interpreting large complex environmental data (Simeonov et al. 2002). These techniques can be used to obtain relationship between parameters and sampling sites, identify the factors and sources influencing groundwater quality and suggest useful tools for both management of water resources and monitoring of groundwater quality (Nosrati and Van Den Eeckhaut 2012). However, in this work, cluster analysis was employed to further explain the possible sources of pollution.
Materials and methods
Study area
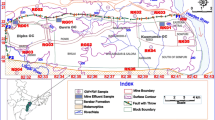
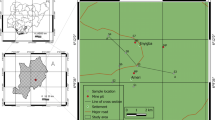
The study area is made of 45 communities spanning the Western and Central regions of Ghana. It lies between latitudes 5′50N and 5′00N and longitudes 1′10W and 1′80W. It spans a total area of about 3607 km2 (WRC 2012) and consists of six (6) districts, namely Lower Denkyira, Assin North, Assin South, Mpohor Wassa East, Shama Ahanta East and Komenda Edna. The climate is sub-equatorial wet, with two rainy seasons (May–July and September–November). The mean annual rainfall is relatively high, about 1500 mm but is also very variable, ranging between 1300 and 1900 mm (WRC 2012). The basin is warm and moist throughout most of the year. Relative humidity is between 70 and 80% throughout the year. In the drier seasons, temperatures are around 26 °C in August and 30 °C in March (WRC 2012). The basin has a drainage area of 23,188 km2. The Pra River and its tributaries constitute a major source of water supply to communities within the basin. The basin is drained by four major tributaries, namely the Ofin, Oda, Anum and Birim. These tributaries merge into the lower Pra which then flows into the Gulf of Guinea through its estuary at Shama (GWSC 1989).
The predominant geological formations underlying the topography of the Pra Basin are mainly the Birimian and Tarkwain both of the pre-cambrian origin and also of Cape Coast granitoid complex and a small percentage of Dixcove granitoid complex.
By constitution the Birimian consists of metamorphosed lava, phyllites, schists and sandstones, whilst the Tarkwaian mainly consists of quartzite, phyllites and schists.
The bulk of the Cape Coast granitoid complex is a granitic to quartz dioritic gneiss, whilst that of Dixcove granitoid complex consists of hornblende granite or granodiorite grading locally into quartz diorite and hornblende diorite (GWSC 1989). The soft constituent rocks like shales and phyllites are found in the valley and lower grounds (GWSC 1989). The basin is principally dominated by the forest ochrosol–oxysol intergrade (Kesse 1985). They also contain more nutrients and are alkaline (GWSC 1989). They have rather variable drainage properties and therefore require protection against erosion (GWSC 1989). In the lower Pra nearer the coast, further classification includes the acid gloisol and coastal savannah soil groups (GWSC 1989) (Fig. 1).
Aquifer characteristics
Borehole depths of the study area are generally shallow ranging between 30 and 96 m and a mean value of 44.42. Borehole yield is generally low and largely variable, ranging from 0.4 to 51.7 m3h−1 and mean value of 4.55 m3h−1 with schist and granite aquifers having relatively higher yields (Tay et al. 2013; Dorleku 2013). The fractures in the rocks are generally open. The granite and schist rocks are exposed, whilst the Birimian and Takwian rocks have thick overburdens. The soils characteristically developed over the same kind of highly weathered parent material with lateritic to clayey top soil layer and thickness which range from 4 to 14 m, which may, however, extend further in some areas. The static water levels of the boreholes generally range from 0.4 to 22.4 m with a mean of 3.7 m (Tay et al. 2013).
Sampling and analysis
Samples were collected from 65 boreholes, making up 130 samples per season in 45 communities. The samples were collected in January 2012 and April 2012 for the dry season and in June and October 2012 for the wet season. Sampling protocols described by Standard Methods for the Examination of Water and Wastewater (APHA 1998) were strictly observed. Samples were collected in 300-ml plastic containers and acidified with concentrated nitric acid (HNO3) to lower the pH to 2. These bottles were previously soaked in 5% HNO3 overnight and thoroughly washed with distilled water. Some amount of water was pumped out of the boreholes before each sample was taken. Temperature, pH and conductivity were measured in the field using Hach Sens ion 156 m. The samples were transported to the laboratory at a temperature of about 4 °C in an ice chest for analysis.
An aliquot of 100 ml of water sample for metal determination is transferred into 125-ml conical flask. 5 ml of concentrated HNO3 was added and evaporated on a hot plate to the lowest volume before precipitation occurs.
Digestion is completed by the appearance of a light-coloured clear solution.
The solution was then filtered through 0.45-µm filter paper and transferred into a 100-ml volumetric flask, cooled and top to the mark for analysis.
A blank was also prepared through the same procedure (APHA 1998).
The concentration of Cu, Fe, Mn, Cd, Zn, Pb was determined using Agilent 240FS atomic absorption spectrophotometer (AAS) by direct aspiration of the water samples into an air acetylene flame, and Al into nitrous oxide acetylene flame. Se and As were determined using a hydride generant attached to the atomic absorption spectrophotometer. Hg was determined using AAS-cold vapour.
Statistical methods
Microsoft Excel 2007 for windows and SPSS 17.0 version were used for the statistical analysis. A descriptive statistics (mean, minimum, median, standard deviation), Student’s t test and hierarchical cluster analysis were achieved.
Cluster analysis
Cluster analysis is an exploratory data analysis tool for solving classification problems. Its object is to sort cases, data or objects (events, people, things, etc.) into groups or clusters (McGarial et al. 2000). The resulting clusters of objects should exhibit high internal (within-clusters) homogeneity and high external (between-clusters) heterogeneity (McGarial et al. 2000). The greater the similarity (or homogeneity) within a group, and the greater the difference between groups, the better or more distinct the clustering. Various types of cluster analysis have been used to analyse water chemistry data for both surface water and groundwater (Güler et al. 2002) Hierarchical agglomerative cluster analysis (HACA) is normally use to examine the water-quality data for spatial and temporal differences.
Güler et al. (2002) described hierarchical cluster analysis as “an efficient means to recognise groups of samples that have similar chemical and physical characteristics.” Hierarchical agglomerative clustering provides intuitive similarity relationships between any one sample and the entire data set and is typically illustrated by a dendrogram (tree diagram) (Singh et al. 2004; Shrestha and Kazama 2007). Euclidean distance method was used for determining distance. In this study, hierarchical CA was performed on the normalised data set using Ward’s method with squared Euclidean distances as a measure of similarity to form a dendrogram to link parameters measured in both dry and wet seasons. The idea is to differentiate parameters into groups of possible anthropogenic and natural geochemical process of temporal variations which in effect goes to proof the contribution of these parameters to the overall quality of the groundwater.
Results and discussion
The summarised results of water (borehole) samples taken from the Pra Basin for each season are presented in Tables 1 and 2, whilst that of the combined season is presented in Table 3.
It can be observed generally from Table 3 that the analytical results for most of the parameters in exception of Hg with a mean of 3.1 µg/l do not suggest heavy metal loadings initially anticipated of a mining area as the Pra Basin. However, the concentration of As, Pb, Cd and Hg as already indicated in some of the boreholes is high enough to pose either physiological or sensory problems, or both if relied on as a source of drinking water. The concentration of these metals in relation to others is illustrated in the box plot in Fig. 2. It can also be seen from the box plot that As, Hg and Se are largely variable and have relatively large range compared to others. Cd, for instance, has its mean very close to its detection limit. The individual trace metals are discussed below.
Several studies have shown that most heavy metals are removed, degraded or accumulated with little downward movement in the vadose zone (Hathhorn and Yonge 1995; Ku and Simmons 1986; Nightingale 1987; Legret et al. 1999; Dierkes and Geiger 1999; Mikkelsen et al. 1997). Crites (1985) suggested five metal removal processes by soil. These are (1) soil surface association, (2) precipitation, (3) occlusion with other precipitates, (4) solid-state diffusion into soil minerals and (5) biological system or residue incorporation. These processes could effectively remove much of the metals and thus protect the underlying groundwater from contamination.
Aluminium
Approximately 17 and 11% of dry and wet seasons, respectively, recorded values above the WHO recommended limit of 200 µg/l. The Lower Pra Basin contains potassium feldspars (KAlSi3O8), albitic feldspars (NaAlSi3O8) and plagioclase (CaAl2Si2O8) (Ahialey et al. 2010). Relatively high values recorded could be as a result of the dissolution of these salts. Generally, Al3+ appears to have only little deleterious effect on humans. Nonetheless, its toxicity has been associated with central nervous system disorders including Alzheimer’s disease and dialysis dementia (Moskowitz et al. 1986). The incidence of discolouration it produces in drinking water and its distribution systems is the greatest problem associated with the metal. The various concentrations do not show any seasonal dependence. There is also no significant difference between the dry and the wet seasons (p > 0.05), as shown in Table 3.
Arsenic
Arsenic concentration in the Basin is generally low with dry season elevations at Atukrom and Adu Banso. The wet season had no value above WHO recommended limit of 10 µg/l (Table 2). These low levels suggest that arsenic presently poses only limited potential physiological problem to the use of groundwater for drinking purposes. The very low concentration of Arsenic in the Basin in spite of the presence of pyrite and arsenopyrite associated with the gold ore suggests the probable effectiveness of the five principal removable mechanisms listed above. Arsenic is a known carcinogen (an agent producing and exciting cancer) and a toxin (Smedley et al. 1995). Long-term, low-level exposure is said to cause skin cancer through the drinking of contaminated water (WHO 1993), and there is suggestive evidence of increasing risk of bladder, kidney, liver and lung tumours as well (Kortatsi 2004). There was no significant difference between the dry and the wet season (p > 0.05).
Cadmium
The dry season recorded values below detection limit for all the samples except for Jerusalem and Achim (Table 2). Approximately 35% of wet season values were above the WHO recommended value of 3.0 µg/l with elevated values of 12 and 16 µg/l at Amoakokrom and Antoabasa, respectively. Higher wet season may suggest anthropogenic influence (Kortatsi 2006). Two boreholes, BH27 and BH28, all at Antoabasa recorded 11 and 16 µg/l, respectively. Ahialey et al. 2010 reported values between 5 and 13 µg/l within the Basin. As already explained, more heavy metals could be leached out in wet season due to the generally higher water table during the season. In addition, more chemicals may be washed out directly from the vadose zoneFootnote 1 by infiltrated rainwater during the wet season (Leung and Jiao 2005).
Cadmium is toxic to man. Cadmium accumulates primarily in the kidneys and has a long biological half-life in humans of 10–35 years. There is some evidence that cadmium may be carcinogenic to experimental animals and it has been implicated in human prostrate carcinoma (WHO 2004; UNEP-GEMS 2000). Absorbed cadmium after acute exposure can cause symptoms such as salivation, difficulty in breathing, nausea, vomiting, pain, anaemia, kidney failure and diarrhoea. Inhalation of cadmium dust or smoke may cause dryness of the throat, headache, chest pain, coughing, increased uneasiness and bronchial complications (Lu et al. 2007). There were significant differences between the dry and wet season values (p < 0.05) indicating possible anthropogenic influence in the boreholes.
Copper
Most of the samples in the dry season recorded values below detection limit with a few points showing significant values and registering a maximum of 83 µg/l at Dweedama (BH10). The wet season recorded a maximum value of 191 µg/l at same sampling point. Generally, copper is not a threat to groundwater pollution in the study area, since all the samples recorded values below the WHO permissible limit of 2000 µg/l. Between 0.04 and 267 µg/l were recorded by Kortatsi (2006) in the Densu Basin. Wet season values at certain points show higher values comparatively to dry season values. Generally, it is expected that wet season values should be lower than that of dry season because of dilution by infiltrating rainwater. However, Leung and Jiao (2005) and Kortatsi (2006) explained that elevated wet season values in groundwater contamination signify possible anthropogenic activity. Hence, copper contribution to groundwater contamination may be due to anthropogenic sources. Comparison of dry and wet seasons shows statistical significant difference (p < 0.05; Table 3), further supporting possible anthropogenic activity. According to NJDEP 2012 bulletin, a decrease in concentration during a drought period may likely represent the dropping of the water table below a “smear zone” of residual contamination as well as seasonal recharge area variations.
Iron
Iron (Fe) occurs naturally from rocks and is found in many surface and groundwater sources at levels ranging 0.3–50 mg/L (WHO 2004). Approximately 15 and 18% of dry and wet season values, respectively, were above WHO recommended limit of 300 µg/l. Iron exists in the water as soluble ferrous ion (Fe2+). When exposed to air, the ferrous ion (Fe2+) is oxidised to the ferric state (Fe3+) which is normally insoluble and precipitates as ferric hydroxide (Appelo and Postma 1999).
This usually causes brown colouration of the water and stains sinks and laundered textiles. The WHO in 1993 limit its concentration in potable water to 300 µg/l because of the aesthetic effect it produces (Kortatsi 2004). However, an upper limit of 1000 µg/l should suffice for most purposes (WHO 1993). The highest concentration was recorded in the wet season at Bremang (2910 µg/l), a typical mining community where mining activity is vigorously being undertaken along the banks of River Abakrampa. Ahialey et al. (2010) reported values between 160 and 780 µg/l for the Basin compared to 5 and 2910 µg/l for the current research. However, no significant difference was observed between the dry and wet seasons (p > 0.05). Iron generally does not appear problematic in the Basin.
Lead
High lead concentration in drinking water may result in metabolic poisoning that manifests in symptoms such as tiredness, lassitude, slight abdominal discomfort, irritation, anaemia and, in the case of children, behavioural changes (WHO 1980). Lead could be found naturally in galena (lead sulphide, PbS), anglesite (lead sulphate, PbSO4), minim (a form of lead oxide with formula Pb3O4), cerussite (lead carbonate, PbCO3) and other minerals. Galena may be the most important natural source.
Generally, the wet season recorded values relatively higher than the dry season values. The mean values were 15.8 and 20.1 µg/l for the dry and wet seasons, respectively. Approximately 43 and 41% of the dry and wet season values were above the WHO (1993) recommended value of 10 µg/l. The relatively high concentrated waters were observed in boreholes in the areas underlain by Lower Birimian and Cape Coast granite (Fig. 1). The high values calls for concern. Although wet season values show relative higher elevation than dry season values, the difference is not statistically significant (p > 0.05). Therefore, the probable contribution of lead to the aquifers could be of natural geochemical processes.
Manganese
Manganese generally dissolves under mildly reducing conditions to produce the mobile divalent manganous ion (Mn2+). When exposed to air, the manganous ion is oxidised to the hydrated oxides that form black colouration and can stain plumbing fixtures and laundered textiles (Hem 1992; Hounslow 1995). Approximately 5 and 11% of the dry and wet season values, respectively, were above the WHO recommended value of 500 µg/l. Elevated values for the dry season were recorded at Senchiem, Ayim, Benso all in the Western region with values of 683, 553 and 540 µg/l, respectively. Wet season elevations occurred in Bremang (BH39 and BH40), Subresu, Semchem (BH49), Achim (BH52, BH53, BH54) with values of 1544, 523, 839, 983, 670, 649, 707 µg/l, respectively. Generally, wet season values were higher than dry season values signifying probable anthropogenic contribution. As already explained, more heavy and trace metals are leached out during the wet season as a result of the generally high water table during the season (Leung and Jiao 2005). It is worth noting that Ayim, Benso, Achim and Bremang are communities involved in small-scale mining activities. However, some heavy metals could be derived naturally. For example, Edmunds and Smedley (2000) suggested that Mn could be released by incongruent or disproportionate reactions from silicate or oxide minerals and emerged as potential residence-time indicators.
Mercury
The toxicity of mercury depends on its chemical form. The form of mercury associated with rocks, and with mining and smelting, thus, likely to be associated with groundwater in the Pra Basin is inorganic mercury. Gold processing using mercury is by a process known generally as mercury amalgamation, which is widely used by Artisanal miners in the Pra Basin. It is reported that both short- and long-term oral exposures can lead to kidney damage and eventually to kidney failure. It can also cause nausea, vomiting, pain, ulceration and diarrhoea (WHO 1980). Approximately, 88 and 42% of the dry and wet season values, respectively, were above the WHO limit of 1.0 µg/l. Dry season values range between 0.01 and 10.1 µg/l at Twifo Mampong (BH37) with the wet season recording values between 0.01 and 16.4 µg/l, with the maximum at Gromsa (BH47), a non-mining community. However, the wet season values suggest less pollution with respect to the dry season. Pra Basin is not known to have mercury in its geological formation. Hence, it is possible mercury contamination is from anthropogenic source via “galamsey”Footnote 2 activity. The relative lower percentage pollution in the wet season might be as a result of rainfall infiltration and percolation resulting in dilution of the aquifers. Studies by Oduro et al. 2012 on surface water including river Pra show mercury levels between 27.59 and 96.95 µg/l. These high values resulting from the indiscriminate use of mercury may be reasons for finding mercury within the basin. Once the polluted rivers, streams and run-offs find their way into the hydrologic path way, the Hg travels along the path way far from the source of pollution. There was significant difference between the dry and wet season values (p < 0.05), which is indicative of possible anthropological pollution.
Selenium
Selenium is present in the Earth’s crust, often in association with sulphur-containing minerals. Selenium is an essential trace element, and foodstuffs such as cereals, meat and fish are the principal source of selenium in the general population. Levels in drinking water vary greatly in different geographical areas but are usually much less than 0.01 mg/l (WHO 2004).
Almost all the boreholes in both dry and wet seasons recorded values below the WHO guideline value of 10 µg/l with majority of them also below detection. Se is used in the production of photocells, semiconductor, stainless steel and glass. However, none of the above industrial activities exists in the basin. This might have accounted for the low levels recorded within the basin.
Zinc
Zn occurs as a natural mineral in many drinking waters. It is an essential dietary nutrient found in virtually all food and potable water in the form of salts or organic complexes (WHO 2004). However, excess zinc can give aesthetic effect on the quality of the water. The WHO recommended limit for the metal is 3000 µg/l. Zinc concentration in the basin ranges between 5.0 and 264 µg/l. Zinc concentrations do not pose any quality problem for groundwater usage in the basin.
Cluster analysis
Results from the cluster analysis as shown in Fig. 3 by the dendrogram were classified into three groups. Group I comprises As, Cd, Cu, Hg, Pb, Se and Zn. This group is made of parameters with the exception of As, Pb and Se which are significantly different in their dry and wet season values as already discussed (Table 3). Their contribution of heavy metal levels in the groundwater was said to be likely anthropogenic because of the significant differences between the dry and the wet seasons (Kortatsi 2006). The presence of lead in this group, even though its contribution is probably from natural geochemical process, could be as a result of its association and occurrence in the environment with Zn, Cd and Cu (Jarup 2003). It also shows that As, Pb and Se are highly correlated with the rest of the group members and therefore their presence in the groundwater could as well be anthropogenic (Tabachnick and Fidell 1996).
Group II (Fig. 3) consists of Mn, Al, Fe with wet season Fe forming a third group but can be conveniently put in this group. The contribution of this group of parameters to the basin is believed to be by natural geochemical process as supported by their p values and therefore in agreement with the initial conclusion that their presence in the groundwater is due to natural geochemical process.
Conclusion
Approximately 84 and 92% of dry and wet season pH values, respectively, recorded values out of the WHO recommended values. The recorded values also show general acidity of boreholes in the study area. More than 95% of pH values in the dry season were either acidic or slightly acidic. All values in the wet season were below a pH of 7. The general acidity of the water samples might be due to drainage of metal-rich rocks in the soil and mine drainage.
Generally, dry season EC values were higher than that of wet season values. The lower values for the wet season can be attributed to dilution by rising water table from rainfall infiltration.
Levels of cadmium, manganese, lead and mercury show values above the WHO guideline values at certain boreholes as well as strong evidence of contribution by anthropogenic sources. The presence of these metals could be due to the exposure of mineralised rocks to air and acidic water through mining activity. Over 40% of boreholes had lead above the WHO recommended value of 10 µg/l. This calls for critical attention. Mercury pollution of the basin is clearly as a result of the indiscriminate prospecting and mining of gold using the amalgamation method. The presence of mercury in groundwater far away from the mining points calls for concern. Approximately 15% of boreholes recorded values above WHO guideline value for Fe and Al, ostensibly posing potential significant aesthetic difficulty to groundwater quality.
There is possibility of acid rock drainage (ARD) occurring in areas where high Fe, Mn/SO42−, and low pH are recorded. Areas with high Fe, but low Mn and SO42− may also indicate ARD since S0 2−4 can easily be transformed by redox reactions and precipitate as sulphides.
The use of hierarchical cluster analysis to delineate the presence of the parameters in the groundwater into anthropogenic and natural geochemical processes further gave credence to the fact that small-scale mining is a contributory factor to the state of groundwater quality in the basin. It also shows that the presence of As, Pb and Se could as well be both anthropogenic and natural geochemical processes (even though their p values > 0.05).
It can be generally concluded that small-scale mining operations negatively impact groundwater quality in the study area with Cd, Cu, Hg and Zn being the principal anthropogenic contributors to the groundwater pollution.
Notes
Vadose zone—the region between a water table and the surface of the earth.
Local parlance for illegal small-scale mining.
References
Ackah M, Agyemang O, Anim AK (2011) Assessment of groundwater quality for drinking and irrigation: the case study of Teiman–Oyarifa Community, Ga East Municipality, Ghana. Proc Int Acad Ecol Environ Sci 1(3–4):186–194
Ahialey EK, Serfo-Armah F, Kortatsi BK (2010) Hydrochemical Analysis of Groundwater in the Lower Pra Basin of Ghana. J. Water Resource and Protection 2010(2):864–871 (Research programme, Project 92/5)
Amegbey NA, Eshun PA (2003) Mercury use and occupational exposure in the Ghanaian small-scale gold mining industry. Ghana Min J 7:54–60
APHA (1998) Standard method for the examination of water and wastewater, 20th edn. American Public Health Association, Washington, p 1998
Appelo CAJ, Postma D (1999) Chemical analysis of groundwater. In: Geochemistry, groundwater and pollution. AA Balkema, Rotterdam
Appiah H (1998) Organization of small-scale mining activities in Ghana. J S Afr Inst Min Metall 98(7):307–310
Aryee BN, Ntibery BK, Atorkui E (2002) Trends in the small scale mining of minerals in Ghana: a perspective on its environmental impact. J Clean Prod 11(2003):131–140
Coakley GJ (1999) The minerals industry of Ghana. In: The U.S. geological survey minerals year book. Area report international, 1007 Africa and Middle East, vol III
Crites RW (1985) Micropollutant removal in rapid infiltration. In: Takashi A (ed) Artificial Recharge of Groundwater. Butterworth Publishers, Boston, pp 579–608
Cunningham WP, Cunningham MA, Saigo B (2005) Environmental science: a global Concern, 8th edn. McGraw-hill, Boston
Dickson KB, Benneh G (1980) A new geography of Ghana. Longman, London
Dierkes C, Geiger WF (1999) Pollution retention capabilities of roadside soils. Water Sci Technol 39(2):201–208
Donkor AK, NarteyVK Bonzongo JL, Adotey DK (2006) Artisanal mining of gold with mercury in Ghana. West Afr J Appl Ecol 9:2–18
Dorleku M (2013) Groundwater contamination with toxic metals through smallscale mining within the Lower Pra Basin, Ghana. M.Phil. thesis, University of Ghana, Legon-Accra, Ghana
Edmunds WM, Smedley PL (2000) Residence time indicators in groundwater: the East Midlands Triassic sandstone aquifer. Appl Geochem 15:737–752
Ghana Water and Sewerage Corporation (GWSC) report, May 1989
Government of Ghana (1989) Small-scale gold mining law 1989, PNDC Law 218, 1989
Güler C, Thyne GD, McCray JE, Turner KA (2002) Evaluation of graphical and multivariate statistical methods for classification of water chemistry data. Hydrogeol J 10:455–474
Hathhorn WE, Yonge DR (1995) The assessment of groundwater pollution potential resulting from stormwater infiltration BMP’s. Final technical report, Research Project T9902, Task 3, Washington State Transportation Center
Hem JD (1992) Study and interpretation of the chemical characteristics of natural water. U.S. Geological Survey Water Supply Paper 2254
Hounslow AW (1995) Water quality data analysis and interpretation. Lewis Publishers, Boca Raton
Jarup L (2003) Hazards of heavy metals contamination. Br Med Bull 68:167–182. https://doi.org/10.1093/bmb/ldg032
Kesse GO (1985) Mineral and rock resources of Ghana. Balkema, Holland
Kortatsi BK (2004) Hydrochemistry of groundwater in the mining area of Tarkwa-Prestea, Ghana. Ph.D. thesis, University of Ghana, Legon-Accra, Ghana
Kortatsi BK (2006) Concentration of trace metals in boreholes in the Ankobra Basin, Ghana. West Afr J Appl Ecol 10:73–87
Ku HFH, Simmons DL (1986) Effects of urban stormwater runoff on groundwater beneath recharge basins on long Island, New York. US Geological Survey Water Resources Investigations Report 85-4088. Prepared in cooperation with Long Island Regional Planning Board, Syosset, New York. USGS, Denver, CO
Legret M, Nicollet M, Miloda P, Colandini V, Raimbault G (1999) Simulation of heavy metal pollution from stormwater infiltration through a porous pavement with reservoir structure. Water Sci Technol 39(2):119–125
Leung CM, Jiao JJ (2005) Heavy metal and trace element distributions in groundwater in natural slopes and highly urbanized spaces in mid-levels area, Hong Kong. Water Res 40(2006):753–767
Lu LT, Chang IC, Hsiao TY, Yu YH, Ma HW (2007) Identification of pollution source of cadmium in soil application of material flow analysis, a case study in Taiwan. Environ Sci Pollut Res 14(1):49–59
McGarial K, Cushman S, Stafford S (2000) Multivariate statistics for wildlife and ecology research. Springer, New York. https://doi.org/10.1007/978-1-4612-1288-1
Meech JA, Veiga MM, Troman D (1998) Reactivity of mercury from gold mining activities in darkwater ecosystems. Ambio 27(2):92–98
Mikkelsen PS, Hafliger M, Ochs M, Jacobsen P, Tjell JC, Boller M (1997) Pollution of soil and groundwater from infiltration of highly contaminated stormwater: a case study. Water Sci Technol 36(8–9):325–330
Moskowitz PD, Coveney EA, Hamilton LD, Kaplan E, Medeiros WH (1986) Identifying human population at risk from acid deposition mobilised materials in drinking water supplies: a preliminary pilot study. Pollution 12:167–172
Nightingale HI (1987) Water quality beneath urban runoff water management basins. Water Resour Bull 23(2):197–205
NJDEP (2012) Technical guidance: site remediation program-monitored natural attenuation. New Jersey Department of Environmental Protection, ver 1.0
Nosrati K, Van Den Eeckhaut M (2012) Assessment of groundwater quality using multivariate statistical techniques in Hashtgerd Plain, Iran. J Environ Earth Sci 65(1):331–344
Oduro WO, Bayitse R, Carboo D, Kortatsi B, Hodgson I (2012) Assessment of dissolved mercury in surface water along the lower basin of river Pra in Ghana. Int J Appl Sci Technol 2(1):228–235
Owens PN, Batalla RJ, Collins AJ, Gomez B, Hicks DM, Horowitz AJ, Kondolf GM, Marden M, Page MJ, Peacock DH, Petticrew EL, Salomons W, Trustrum NA (2005) Fine-grained sediment in river systems: environmental significance and management issues. River Res Appl 21:693–717
Pfeiffer WC, Larceda LD (1988) Mercury input into the Amazom Region, Brazil. Environ Technol Lett 9:325–330
Shoko DSM, Love D (2005) Gold panning legislation in Zimbabwe: what potentials for sustainable management of river resources. In: Mathew K, Nhapi I (eds) Water and wastewater management for development countries. IWA Water and Environmental Management Series, IWA Publishing, London, pp 499–512
Shrestha S, Kazama F (2007) Assessment of surface water quality using multivariate statistical techniques: a case study of the Fuji river basin, Japan. Environ Model Softw 22:464–475. https://doi.org/10.1016/j.envsoft.2006.02.001
Simeonov V, Einax JW, Stanimirova I, Kraft J (2002) Environmetric modelling and interpretation of river water monitoring data. Anal Bioanal Chem 374:898–905
Singh KP, Malik A, Mohan D, Sinha S (2004) Multivariate statistical techniques for the evaluation of spatial and temporal variations in water quality of Gomti River (India): a case study. Water Res 38:3980–3992. https://doi.org/10.1016/j.watres.06.011
Smedley PL, Ednunds WM, West JM, Gardner SJ, Pelig-Ba KB (1995) Vulnerability of shallow groundwater quality due to natural geochemical environment. Health problems related to groundwater in the Obuasi and Bolgatanga Areas, Ghana. Report prepared for ODA under the ODA/BGS technology development
Tabachnick B, Fidell L (1996) Using multivariate statistics. Harper Collins College Publishers, New York
Tay C, Hayford E, Hodgson IO, Kortatsi BK (2013) Hydrochemical appraisal of groundwater evolution within the Lower Pra Basin, Ghana: a hierarchical cluster analysis (HCA) approach. Environ Earth. https://doi.org/10.1007/s12665-014-3644-4
United Nations Environment Programme (UNEP) (2000) Global environment monitoring system/water programme
Water Resources Commission (WRC) (2008) Ghana’s water resources. http://www.wrcgh.org/ghanaswaterresources.html. Accessed 12 Aug 2016
Water Resources Commission (WRC) (2012) Pra River Basin-integrated water resources management plan, June 2012
World Health Organisation (WHO) (1980) Recommended health-based limits in occupational exposure to trace elements
World Health Organisation (WHO) (1993) Guidelines for drinking water quality. Revision of the 1984 guidelines. Final task group meeting. Geneva 21–25 Sept 1992
World Health Organisation (WHO) (2004) Guidelines for drinking-water quality, vol 1, 3rd edn. Geneva
Funding
Funding was provided by CSIR-WRI.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Dorleku, M.K., Nukpezah, D. & Carboo, D. Effects of small-scale gold mining on heavy metal levels in groundwater in the Lower Pra Basin of Ghana. Appl Water Sci 8, 126 (2018). https://doi.org/10.1007/s13201-018-0773-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13201-018-0773-z