Abstract
We studied the distribution of proto- and metazooplankton coupled with environmental factors in the coast area around Kneiss Islands (Central Mediterranean Sea). Zooplanktonic communities were sampled during summer 2009 and summer 2010 at three stations. Our results showed difference in suspended matter concentrations between summer 2009 (92.88 ± 7.15 mg L−1) and summer 2010 (47.37 ± 23.12 mg L−1). Large variations in the N/P ratio were recorded (6.94–36.76) due to the direct influence of the variability in concentration of both the dissolved inorganic nitrogen and dissolved inorganic phosphate components of the ratio. Ciliates abundance peaked in summer 2009 and was 3 times more abundant than summer 2010. Ciliates community composition was dominated by loricate ciliates (75% of total ciliates) in summer 2009 and naked ciliates (56% of total ciliates) in summer 2010. Copepods were the most abundant metazooplankton present during the entire study period, comprising 30–96% of the total metazooplankton community. Small planktonic copepods reached important abundance, particularly oithonids, were found to largely dominate copepods community in both summer 2009 (Oithona nana, 45% of total copepods) and summer 2010 (Oithona similis, 22% of total copepods). The small planktonic species Paracalanus parvus (54% of total copepods) was abundant during summer 2010. The results also indicate that (1) ciliates abundance was very low, showing a possible predation by copepods and also by heterotrophic dinoflagellates, (2) copepods capable to complete a top-down control on phytoplankton and ciliates, with preference to ciliates more than diatoms of similar size and shape and (3) the resistance of loricate ciliates compared to naked ciliates may be explained by their capacity to escape grazing due to the existence of a protective lorica.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The coast of Sfax is a part of the eastern Mediterranean sea and is located in south of Tunisia (Rekik et al. 2012). It is bounded by the Kneiss Islands in the northeast, which is composed of 4 little islands (El Bessila, El Hajar, El Laboua and El Gharbia) with very low landforms of calcareous sand stone. El Bessila is occupied by sebkha, chotts and, especially, maritime marshes (Gueddari and Oueslati 2002). Everywhere the soft material of the island’s shores is the victim of erosion. These islands are uninhabited (Mosbahi et al. 2015). The islands encourage the presence of many halophilous species, covering over 80% of the soil surface. These vast stretches are a favourite site for aquatic avifauna, basically migratory and wintering birds, which occupy all the niches in the various available water levels. Because it lies in pre-Saharan Tunisia, this site is a major stopover for migratory birds. There are a great many birds: 70% of Tunisia’s birds winter in the Kneiss Islands, and there may be more than 100,000 of them (Hamza et al. 2015). Over 75% of the marine plant populations are marine phanerogams, including several thousand hectares of Cymodocea lawns (Mosbahi et al. 2016a). The islands are colonized by the seagrass Zostera noltei, protected species listed in the “IUCN Red List” of threatened species in Mediterranean Sea, as characterizing a diversified habitat requiring monitoring and protection (Mosbahi et al. 2016b). The originality of the site’s appearance is a result of the existence of a very big sandy-silty estran, crossed by channels. The Kneiss Islands were declared as a “Nature Reserve” in 1993. These islands have been a SPAMI “Specially Protected Area of Mediterranean Importance” since 2001 because it is having one of the Mediterranean’s biggest estran ecosystems with exceptional avifauna (Mosbahi et al. 2016b). The archipelago was stated as an “Important Bird Area” (IBA) in 2003 and a “RAMSAR Site” in 2007 (Mosbahi et al. 2016b). Yet, the Kneiss Islands environment is suffering from considerable pollution through hypertrophication, basically due to the consequences of the phosphate processing industry and the dumping of hydrocarbons (Rekik et al. 2012). The presence of hundreds of boatless fishermen in the islands, especially el Bessila, seriously harms the conservation of the environment, since it greatly disturbs the nesting birds or even prevents them making any attempt at nesting (M’Rabet 1995). Marine turtles present in the area are caught sporadically. For the entire marine coastal area, the scraping of the seabed is very likely to lessen the area’s biodiversity (Galil 2000).
Mosbahi et al. (2015, 2016a, b) presented a research that investigated the spatial and seasonal structure of the intertidal macrozoobenthic communities of the area around the Kneiss Islands. However, data concerning the spatial and inter-annual distribution of ciliates and zooplankton assemblages coupled to environmental factors in the Kneiss Islands coast were scarce. Our objective was (1) to investigate the dynamic and diversity of the planktonic ciliates and metazooplankton through summer 2009 and summer 2010; (2) to identify the role of the main abiotic factors that determine the protozooplankton and metazooplankton communities structure and functioning; and (3) to study the spatial distribution of ciliates and metazooplankton during summer in relation to its potential prey such as phytoplankton. To the best of our knowledge, there have been no previous comprehensive field studies that include all of the same parameters found in our study.
Materials and methods
Study site and sampling procedures
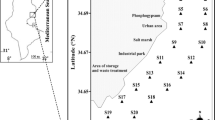
Nutrients, phytoplankton, ciliate and metazooplankton samples were collected in July 2009 and July 2010. Water samples were collected on 3 stations around the Kneiss Islands coast (Fig. 1). Seawater samples for physico-chemical analyses, phytoplankton and ciliate examination were collected from the surface water with a Van Dorn-type closing bottle at each station. Metazooplankton was collected using a cylindro-conical net (30 cm aperture, 100 cm high, and 100 μm mesh size). Nutriment samples (120 mL) were stored immediately in the dark at 20 °C. Phytoplankton samples (1 L) were preserved with Lugol iodine solution (4%) for enumeration (Bourrelly 1985). Metazooplankton samples were preserved in 2% buffered formaldehyde solution and were stained with rose Bengal to facilitate dissection. Plankton samples were kept at low temperature (4 °C) in the dark until analysis.
Physico-chemical variables
Physical parameters (temperature, salinity, and pH) were measured immediately after sampling using a multi-parameter kit (Multi 340 i/SET). Suspended matter concentrations were measured using the dry weight of the residue after filtration of 0.5 L of seawater onto Whatman GF/C membrane filters. Chemical parameters (nitrite, nitrate, ammonium, orthophosphate, silicate, total nitrogen and total phosphate) were analysed with a Bran and Luebbe type 3 autoanalyzer.
Plankton enumeration
Phytoplankton and ciliates samples (50 mL) were analysed under an inverted microscope after 24–48 h settling using the Utermöhl method (Utermöhl 1958). Zooplankton enumeration was performed under a vertically mounted deep-focus dissecting microscope (Olympus TL 2). Plankton species identification was made according to various keys (Rekik et al. 2012, 2013a, b, 2015a, b, c).
Statistical analysis
The data recorded in this study were submitted to a normalized principal component analysis (PCA) using XLstat (Dolédec and Chessel 1989). Simple log (x + 1) transformation was applied to data in order to correctly stabilize variance (Frontier 1973). Means and standard deviations (SD) were reported when appropriate. The potential relationships between variables were tested with Pearson’s correlation coefficient. One-way ANOVA employing XLstat software followed by a post hoc evaluation using Tukey’s analysis was used to recognize major variations among summer 2009 and summer 2010.
Results
Environmental parameters
The physical characteristics of the study are summarized in Table 1. The average of the water temperature ranged between 31 and 38 °C. The lowest temperature was detected at station 3 in summer 2009 and in stations 1 and 3 in summer 2010. The highest temperature was recorded at station 1 during summer 2009. The mean temperature values, which varied between 35.33 ± 3.79 °C and 31.33 ± 0.58, showed similar variations in both summer 2009 and summer 2010 during the survey period. The salinity ranged from 37.5 to 39 psu recorded in stations 2 and 1 during summer 2010 and summer 2009, respectively (Table 1). The pH was relatively stable and distributed homogeneously throughout the monitoring stations varying from 8 to 8.4 observed in all sampling area (Table 1). The mean pH values were usually alkaline, suggesting a pronounced photosynthetic activity. The mean suspended matter concentrations showed an important difference between summer 2009 (92.88 ± 7.15 mg L−1) and summer 2010 (47.37 ± 23.12 mg L−1). The highest value was recorded in station 2 from summer 2009 and the lowest in summer 2010 (28.10 mg L−1 in summer 2010) (Table 1).
The chemical variables analysed during this study are summarized in Table 1. Nitrate and nitrite concentrations did not show marked changes between summer 2009 and summer 2010. On the contrary, during summer 2010, ammonium concentration showed a significant increase in stations 1 (8.04 µM) (Table 1). Overall, the concentration of total nitrogen was not changed from summer 2009 to summer 2010 of the coastal area. Summer 2009 total nitrogen concentration ranged from 12.35 (station 2) to 15.20 µM (station 3). In summer 2010, total nitrogen concentration was normally, ~ 14 µM throughout the study period (Table 1). The mean value of orthophosphate concentration was 0.29 µM, showed similar variations in both summer 2009 and 2010 (Table 1). When considering total phosphate concentrations, values were 8 times that of orthophosphate concentration in the summer 2009 and up to 21 times in the summer 2010 (Table 1). The N/P: DIN (DIN = NO2− + NO3− + NH4+) to DIP (DIP = PO43−) ratio ranged from 6.94 in summer 2009 (station 3) to 36.76 in summer 2010 (station 1). However, the DIN/DIP ratio was generally higher than the Redfield ratio (16), with the exception of station 3 (6.94 in summer 2009 and 15.60 in summer 2010) (Table 1). These averages were more important than the Redfield ratio (16), suggesting a potential P limitation. The largest variation of silicate concentration (4.95 ± 2.48–12.05 ± 1.24 µM) was observed during summer 2009 and summer 2010, respectively (Table 1).
Ciliates, community diversity and dynamics
Ciliates abundance ranged from 2.00 × 102 to 15.00 × 102 cells L−1 (3.00 ± 1.00 × 102 cells L−1 in summer 2010, 9.33 ± 4.93 × 102 cells L−1 in summer 2009). Ciliates proliferated exclusively at station 3; the highest ciliates abundance (15.00 × 102 cells L−1) was recorded during summer 2009 (Fig. 2). In terms of inter-annual abundance distribution, the majority of ciliates were found in summer 2009. Loricate ciliates summer 2009 abundance varied from 4 to 11 × 102 cells L−1 recorded at stations 1 and 3, respectively. In summer 2010, the variation of loricate ciliates abundance followed a similar trend to that of summer 2009, with an average value of 1.33 ± 1.53 × 102 cells L−1 less important than summer 2009, 7.00 ± 3.61 × 102 cells L−1. Loricate ciliates abundance peaked at the same time, in summer 2009 (11.00 × 102 cells L−1) and summer 2010 (3.00 × 102 cells L−1), at station 3 (Fig. 2). Loricate ciliates made the most abundant group during summer 2009 (75% of total ciliates), but, in summer 2010, the contribution of loricate ciliates is only 44% of total ciliates. Naked ciliates were observed at all stations, whereas they were poorly represented in terms of abundance. In terms of inter-annual distribution, the majority of other ciliates were found in summer 2010 (56% of total ciliates abundance). Naked ciliates abundance varied from 102 to 4.00 × 102 cells L−1; the highest naked ciliates abundance was observed in station 3 during summer 2009. The inter-annual mean of naked ciliates abundance was 2.33 ± 1.53 × 102 and 1.67 ± 0.58 × 102 cells L−1 in summer 2009 and summer 2010, respectively (Fig. 2).
The ciliates community consisted of 15 taxa (12 species in summer 2009 and 5 species in summer 2010) belonging to 10 genera. Figure 3 reports the abundances of all the observed taxa during summer 2009 and summer 2010. Clear differences in ciliates diversity and composition were found from year to year and between stations. Naked ciliates were the most diverse ciliates group with 8 species (5 and 4 taxa summer 2009 and summer 2010). The genus Tintinnopsis was dominant among ciliates (4 taxa), followed by Euplotes and Strombidium and (8 taxa) (Fig. 3). Tintinnopsis beroidea was the most important species and peaked in station 3 at both sampling periods (5.00 × 102 cells L−1, summer 2009 and 3.00 × 102 cells L−1, summer 2010). Among the species collected, Favella ehrenbergi and Tintinnopsis sp. were found with important abundance. There were 10 species specific of summer 2009 (Favella ehrenbergi, Helicostomella subulata, Tintinnopsis aperta, Tintinnopsis radix, Tintinnopsis sp., Undella sp., Colpoda sp., Euplote sp., Leegardiella sol and Lohmanniella oviformis) and 3 species for summer 2010 (Aspidisca lynceus, Strombidium capitatum and Strombidium conicoides). Some ciliates taxa were omnipresent at all study, and among these ciliates, Euplote charon (Fig. 3).
Zooplankton, community diversity and dynamics
The total metazooplankton abundance varied from 21.50 × 102 (station 3, Summer 2009) to 123.35 × 102 ind m−3 (station 2, summer 2009), the highest mean abundance being recorded in summer 2010 (67.64 ± 15.08 × 102 ind m−3) (Fig. 4). Metazooplankton assemblages along the coast of the Kneiss Islands were dominated by copepods accounting for 45 and 84% of the total metazooplankton abundance during summer 2009 and 2010, respectively. The summer 2009 distribution of copepods (29.72 ± 8.45 × 102 ind m−3) illustrated a high cyclopoids density (17.69 × 102 ind m−3) at station 1 and was associated with Oithonidae aggregations. Abundances of copepods, copepods nauplii, cyclopoids and calanoids peaked at the same time, in summer 2010 at station 1. High abundances of calanoids were recorded during summer 2010. Calanoids clearly dominated the copepods during summer 2010, accounting for 50% of total copepods abundances. Low numbers of copepods nauplii were observed, ranging between 0 and 8.74 × 102 ind m−3 (1.29 ± 1.12 × 102 ind m−3, summer 2009 and 7.40 ± 1.72 × 102 ind m−3, summer 2010). The inter-annual distribution of Harpacticoids and Poecilostomatoids was slightly greater in summer 2009. Harpacticoids varied from 1.83 to 10.30 × 102 ind m−3 (4.77 ± 3.23 × 102 ind m−3 in summer 2009 and 4.72 ± 4.84 × 102 ind m−3 in summer 2010) and Poecilostomatoids from 0 to 1.89 × 102 ind m−3 (0.88 ± 0.95 × 102 ind m−3 in summer 2009 and 0.90 ± 0.20 × 102 ind m−3 in summer 2010) (Fig. 4), following the copepods community throughout the study period, reaching only low abundances. Other metazooplankton clearly dominated the metazooplankton community during summer 2009, accounting for 55% of total metazooplankton abundances. Other metazooplankton abundance varied from 0.79 × 102 (station 3) to 85.88 × 102 ind m−3 (station 2), the highest mean abundance being recorded in summer 2009 (37.03 ± 43.92 × 102 ind m−3) (Fig. 4).
A summary of the copepods species and other metazooplankton groups observed throughout the study period is given in Fig. 5. Metazooplankton composition was significantly different among the 2 years and stations. A total of 14 copepods families were found at all stations, with Oithonidae dominating the copepods community, among which Oithona nana and Oithona similis were the most abundant species, representing 45 and 22% of the total copepods presence in summer 2009 and summer 2010, respectively. Copepods richness was more pronounced in summer 2009 (16 species) than in summer 2010 (13 species) (Fig. 5). Copepods consisted particularly of Euterpina acutifrons (station 2 in summer 2009 and station 3 in summer 2010), Centropages typicus (station 1, summer 2009) and Paracalanus parvus (station 1, summer 2010) (Fig. 5).
Statistical analysis
Principal component analysis (PCA) allowed discrimination of four groups around the components of the F1 and F2 axes (Fig. 6), explaining 100% of the variance. The F1 axis, explaining 65.10% of the variability of abiotic and biotic parameters, positively selected group G1 composed of NO3−, NH4+, N/P ratio, total phytoplankton, total zooplankton, copepods, other zooplankton, calanoids and harpacticoids. The F2 axis, representing 34.90% of the variability, positively selected group G2 comprising physical factors such as temperature and salinity correlated with cyclopoids abundance. G3 comprised T-N, copepods nauplii and other ciliates. The group G4 was formed by pH, suspended matter, NO2−, PO43−, T-P, Si(OH)4, total ciliates, tintinnids and poecilostomatoids. This combination was selected in summer 2009 (Fig. 6).
During summer 2010, the PCA distinguished between four groups surrounding the F1 and F2 component axes thus explaining 100% of the variance. The axes selected a group G1 comprising the biological parameters (total phytoplankton, total zooplankton, copepods, other zooplankton, copepods nauplii, cyclopoids and calanoids) and several physico-chemical variables (pH, NH4+, T-P and N/P ratio). F1 component axis, which extracted 61.05% of the variability, selected positively the group G2, with total ciliates, tintinnids and harpacticoids correlating to abiotic parameters (salinity, NO3−, PO43− and Si (OH)4). The gradient along the F1 axis is mainly due to suspended matter and poecilostomatoids (selected negatively in G3) and to temperature, NO2−, T-N and other ciliates (selected negatively in G4) (Fig. 6).
Discussion
The present study is the first examining the spatial distribution of ciliates and zooplankton communities in the shallow coastal waters around Kneiss Islands coupling to nutrients and phytoplankton abundance during summer 2009 and summer 2010. Our results demonstrated distinct inter-annual contrasts. Ciliates and zooplankton assemblages in coastal ecosystems are controlled by a combination of environmental and biological factors.
During this study, the high values of temperature and salinity were typical of arid to semiarid zones (Elloumi et al. 2015). Suspended matter concentrations recorded in summer 2009 (92.88 ± 7.15 mg L−1) were higher than those founded in summer 2010 (47.37 ± 23.12 mg L−1). The striking difference of the suspended matter concentrations levels can logically be attributed to the shallowness of the sampled stations and the spatial distribution of polluting elements and that may result in resuspension of tiny particles with, for instance, tidal action (Ben Salem et al. 2015). Large variations in the N/P ratio were recorded (6.94–36.76) due to the direct influence of the variability in concentration of both the dissolved inorganic nitrogen and dissolved inorganic phosphate components of the ratio. The average value of the N/P ratio was generally higher than the Redfield (16) ratio in both summer 2009 and summer 2010. However, large variations in the N/P ratio were observed due to the direct influence of the variability in concentration of both the N and P components of the ratio. These results may be explained by phytoplankton’s rapid consumption (Rekik et al. 2016), and the importance of nitrogen availability may be caused by atmospheric deposition (Liu et al. 2015).
In this study, there was an important difference in ciliates abundance between years; on the other hand, the difference influences the ciliates composition and distribution. For example, in terms of annual scale, ciliates abundance peaked in summer 2009 and was 3 times more abundant than summer 2010. With regard to the structural composition, the ciliates community at Kneiss Islands coast was dominated by loricate ciliates (75% of total ciliates) in summer 2009 and naked ciliates (56% of total ciliates) in summer 2010. At the coastal waters around Kneiss Islands, loricate ciliates demonstrated a clear inter-annual pattern: high values in summer 2009 and low values in summer 2010 with an obvious peak in station 3 throughout the study period. Loricate ciliates counted for a large percentage (0–73%) of the total ciliates. Their density seemed to be associated to their morphology that they can escape grazing due to the existence of a protective lorica (Abboud-Abi Saab 2008), their tolerance of marine habitats stress and high flexibility to eutrophic conditions. With regard to the structural composition, loricate ciliates population at the coast of Kneiss Islands was dominated by the genus Tintinnopsis. This genus has usually been reported to be the dominant planktonic ciliates in both temperate and tropical coastal systems (Dolan 2006). The most abundant species was Tintinnopsis beroidea; this species is relatively common in coastal ecosystems and in particular in the north coast (Rekik et al. 2015b) and south coast (Rekik et al. 2015c) of Sfax. The dominance of the agglutinated species T. beroidea appears to be related to the availability of particles to construct the lorica (Rakshit et al. 2015). This result suggests that this species performed better than other taxa probably owing to more flexible adaptive strategies, to particular environmental factors (temperature, salinity) and the shallowness of the water sampling (Kchaou et al. 2009).
Small planktonic copepods reached important abundance throughout the study period. The advantage of the small taxa bearing egg sacs is to keep eggs against predators until they hatch and make nauplii (Ben Ltaief et al. 2015). Small copepods, particularly oithonids, were found to largely dominate copepods community in both summer 2009 (Oithona nana, 45% of total copepods) and summer 2010 (Oithona similis, 22% of total copepods). This appears to be a common feature in the north coast (Rekik et al. 2012) and south coast (Ben Salem al. 2015) of Sfax, in the Gulf of Gabes (Ben Ltaief et al. 2015) and in the north of Tunisia (Daly Yahia et al. 2004). Other studies reported a very important contribution of small copepods over the Balearic Sea (Fernandez de Puelles et al. 2003), the Adriatic Sea (Vidjak et al. 2007) and the Aegean Sea (Zervoudaki et al. 2007). The dominance of small species, particularly the genus Oithona, in salty and warm coastal waters has also been reported in the bay of Tunis (Daly Yahia et al. 2004) and in the Lagoon of Tunis (Annabi-Trabelsi et al. 2006) (north of Tunisia). The dominance of Oithona nana in various marine ecosystems was inferred owing to its adaptive strategies (Riccardi and Mariotto 2000) allowing it to tolerate environmental perturbations (Gallienne and Robins 2001). Oithona similis has been recognized as the most ubiquitous copepod species in the world ocean (Gallienne and Robins 2001). This small species revealed clear eurythermal and euryhaline distribution (Bel Hassen et al. 2008). The Kneiss Islands coast was also distinguished by the prevalence of the small planktonic species Paracalanus parvus (27.52 × 102 ind m−3) in summer 2010. This species was known by omnivorous diet (Turner 1991).
During this study, the ciliates abundance was very low, showing a possible predation by copepods and also by heterotrophic dinoflagellates (Jeong et al. 2010). Copepods represent an important metazooplankton group capable to complete a top-down control on phytoplankton (r = 0.91, n = 24, p = 0.05) and ciliates (r = 0.68, n = 24, p = 0.05) communities (Zervoudaki et al. 2007). Similar results reported the strong top-down control of copepods in regulating phytoplankton and ciliates populations in the eastern Mediterranean Sea (Siokou-Frangou et al. 2002). Moreover, the resistance of loricate ciliates compared to naked ciliates may be explained by their capacity to escape grazing due to the existence of a protective lorica (Abboud-Abi Saab 2008). Oithona nana is a species known to be omnivorous (Lampitt and Gamble 1982), opportunistic (Lam-Hoai and Rougier 2001) and capable of feeding on small prey. Oithona similis is omnivorous feeding on heterotrophic dinoflagellates, ciliates and naupliar stages of copepods (Turner 2004). Indeed, this copepod has preference to ciliates more than diatoms of similar size and shape (Castellani et al. 2008).
Conclusion
The proto- and metazooplankton abundance showed clear variations along the coastal stations during summer cruises conducted in years 2009 and 2010. This study indicates that ciliates were largely dominated by loricate ciliates in summer 2009 and naked ciliates in summer 2010, while copepods were the most numerous metazooplankton, including Oithona nana and Oithona similis which was spread abundantly along the coast during summer 2009 and summer 2010, respectively. The trophic interplay between phytoplankton, ciliates and copepods suggests that copepods were capable to complete a top-down control on phytoplankton and ciliates communities. On the other hand, trophic interplay between phytoplankton, ciliates and copepods suggests that abiotic parameters and nutrients were also involved in the environmental forcing of the summer proto- and metazooplankton dynamics around Kneiss Islands.
References
Abboud-Abi Saab M (2008) Tintinnids of the Lebanese coastal waters (Eastern Mediterranean). CNRS-Lebanon/UNEP/MAP/RAC/SPA, Lebanon
Annabi-Trabelsi N, Daly-Yahia MN, Romdhane MS, Ben Maïz N (2006) Seasonal variability of planktonic copepods in Tunis North Lagoon (Tunisia, North Africa). Cah Biol Mar 46:325–333
Bel Hassen M, Drira Z, Hamza A, Ayadi H, Akrout F, Issaoui H (2008) Summer phytoplankton pigments and community composition related to water mass properties in the Gulf of Gabes. Estuar Coast Shelf Sci 77:645–656
Ben Ltaief T, Drira Z, Hannachi I, Bel Hassen M, Hamza A, Pagano M, Ayadi H (2015) What are the factors leading to the success of small planktonic copepods in the Gulf of Gabes, Tunisia? J Mar Biol Assoc UK 95:747–761
Ben Salem Z, Drira Z, Ayadi H (2015) What factors drive the variations of phytoplankton, ciliate and mesozooplankton communities in the polluted southern coast of Sfax, Tunisia?. Sci Pollut Res, Environ. https://doi.org/10.1007/s11356-015-4416-8
Bourrelly P (1985) Introduction to systematic. The blue and red algae, vol II. Edité par Société Nouvelle des Editions Boubée, Paris, p 450
Castellani C, Irigoien X, Mayor DJ, Harris RP, Wilson D (2008) Feeding of Calanus finmarchicus and Oithona similis on the microplankton assemblage in the Irminger Sea, North Atlantic. J Plankton Res 30:1095–1116
Daly Yahia MN, Souissi S, Daly Yahia Kefi O (2004) Spatial and temporal structure of planktonic copepods in the Bay of Tunis (southwestern Mediterranean Sea). Zool Stud 43:366–375
Dolan JR (2006) Comparing taxonomic and morphological biodiversity of tintinnids (planktonic ciliates) of New Caledonia. Limnol Oceanogr 2:950–958
Dolédec S, Chessel D (1989) Rythmes saisonniers et composantes stationnelles en milieu aquatiqueII. Prise en compte et élimination d’effets dans un tableau faunistique. Acta Oecol Oecol Gen 10:207–332
Elloumi J, Drira Z, Guermazi W, Hamza A, Ayadi H (2015) Space-time variation of ciliates related to environmental factors in 15 nearshore stations of the Gulf of Gabes (Tunisia, Eastern Mediterranean Sea). Mediterr Mar Sci. https://doi.org/10.12681/mms.792
Fernandez de Puelles ML, Gras D, Hernandez Leon S (2003) Annual cycle of zooplankton biomass, abundance and species composition in the neritic area of the Balearic Sea, Western Mediterranean. Mar Ecol PSZNI 24:123–139
Frontier S (1973) Etude statistique de la dispersion du zooplancton. J Exp Mar Biol Ecol 12:229–262
Galil BS (2000) A sea under siege: alien species in the Mediterranean. Biol Invasions 2:177–186
Gallienne CP, Robins DB (2001) Is Oithona the most important copepod in the world’s oceans? J Plankton Res 23:1421–1432
Gueddari M, Oueslati A (2002) Le site de Kneiss, Tunisie: géomorphologie et aptitudes à l’aménagement. In: Scapini F (ed) Recherche de Base pour une Gestion Durable des Ecosystèmes Sensibles Côtiers de la Méditerranée. Istituto Agronomico per l’Oltremare, Italie, pp 63–71
Hamza F, Hammouda A, Selmi S (2015) Species richness patterns of waterbirds wintering in the gulf of Gabès in relation to habitat and anthropogenic features. Estuar Coast Shelf Sci 165:254–260
Jeong HJ, Yoo YD, Kim JS, Seong KA, Kang NS, Kim TH (2010) Growth, feeding and ecological roles of the mixotrophic and heterotrophic dinoflagellates in marine planktonic food webs. Ocean Sci J 45:65–91
Kchaou N, Elloumi J, Drira Z, Hamza A, Ayadi H, Bouain A, Aleya L (2009) Distribution of ciliates in relation to environmental factors along the coastline of the Gulf of Gabes, Tunisia. Estuar Coast Shelf Sci 83:414–424
Lam-Hoai T, Rougier C (2001) Zooplankton assemblages and biomass during a 4-period survey in a Northern Mediterranean coastal lagoon. Water Res 35:271–283
Lampitt RS, Gamble JC (1982) Diet and respiration of the small planktonic marine copepod Oithona nana. Mar Biol 66:185–190
Liu X, Huang B, Huang Q, Wang L, Ni X, Tang Q (2015) Seasonal phytoplankton response to physical processes in the southern Yellow Sea. J Sea Res 95:45–55
M’Rabet R (1995) Les engins de pêche et les ressources halieutiques. Notes de l’Institut National Scientifique et Technique d’Océanographie et de Pêche de Salammbô 6:1–29
Mosbahi N, Boudaya L, Dauvin JC, Neifar N (2015) Spatial Distribution and Abundance of Intertidal Benthic Macrofauna in the Kneiss Islands (Gulf of Gabès, Tunisia). Cah Biol Mar 56:319–328
Mosbahi N, Pezy JP, Dauvin JC, Neifar L (2016a) Spatial and temporal structures of the macrozoobenthos from the intertidal zone of the Kneiss Islands (Central Mediterranean Sea). Open J Mar Sci 6:223–237
Mosbahi N, Pezy JP, Dauvin JC, Neifar L (2016b) Short-term impact of bait digging on intertidal macrofauna of tidal mudflats around the Kneiss Islands (Gulf of Gabès, Tunisia). Aquat Living Resour 28:111–118
Rakshit D, Ganesh S, Sarkar SK (2015) Choreotrich ciliate tintinnid (Protozoa: Ciliophora) in a tropical meso–macrotidal estuary, eastern part of India. Stud Mar Sci, Reg. https://doi.org/10.1016/j.rsma.2015.06.003
Rekik A, Drira Z, Guermazi W, Elloumi J, Maalej S, Aleya L, Ayadi H (2012) Impacts of an uncontrolled phosphogypsum dumpsite on summer distribution of phytoplankton, copepods and ciliates in relation to abiotic variables along the near-shore of the southwestern Mediterranean coast. Mar Pollut Bull 64:336–346
Rekik A, Maalej S, Ayadi H, Aleya L (2013a) Restoration impact of an uncontrolled phosphogypsum dump site on the seasonal distribution of abiotic variables, phytoplankton and zooplankton along the near shore of the south-western Mediterranean coast. Environ Sci Pollut Res. https://doi.org/10.1007/s11356-012-1297-y
Rekik A, Denis M, Aleya L, Maalej S, Ayadi H (2013b) Spring plankton community structure and distribution in the north and south coasts of Sfax (Tunisia) after north coast restoration. Mar Pollut Bull 67:82–93
Rekik A, Denis M, Maalej S, Ayadi H (2015a) Spatial and seasonal variability of pico-, nano- and microphytoplankton at the water-sediment interface in the north coast of Sfax, Eastern Mediterranean Sea. Environ Sci Pollut Res. https://doi.org/10.1007/s11356-015-4811-1
Rekik A, Denis M, Maalej S, Ayadi H (2015b) Planktonic ciliates in relation to abiotic variables on the north coast of Sfax after environmental restoration: species composition, and abundance-biomass seasonal variation. J Oceanogr Res Data 8:1–16
Rekik A, Elloumi J, Charri D, Ayadi H (2015c) Phytoplankton and ciliate communities’ structure and distribution in a stressed area of the south coast of Sfax, Tunisia (Eastern Mediterranean Sea). J Mar Freshw Res. https://doi.org/10.1071/mf15057
Rekik A, Ben Salem Z, Ayadi H, Elloumi J (2016) Spring phytoplankton variability along a south coast of Sfax at the water-sediment interface (Tunisia, Eastern Mediterranean Sea). J Coast Life Med 4:121–127
Riccardi N, Mariotto L (2000) Seasonal variations in copepod body length: a comparison between different species in the lagoon of Venice. Aquat Ecol 34:243–252
Siokou-Frangou I, Bianchi M, Christaki U, Christou ED, Giannakourou A, Gotsis O, Ignatiades L, Pagou K, Pitta P, Psarra S, Souvermezoglou E, Van Wambeke F, Zervakis V (2002) Carbon flow in the planktonic food web along a gradient of oligotrophy in Aegean Sea (Mediterranean Sea). J Mar Syst 33:335–353
Turner JT (1991) Zooplankton feeding ecology: do co-occurring copepods compete for the same food? Rev Aquat Sci 5:101–195
Turner JT (2004) The importance of small planktonic copepods and their roles in pelagic marine food webs. Zool Stud 43:255–266
Utermöhl H (1958) Toward the improvement of the quantitative phytoplankton method. Mitteilungen Int Vereiningung Limnol, Deutsch, pp 1–38
Vidjak O, Bojanic N, Kuspilic G, Gladan ZN, Ticina V (2007) Zooplankton community and hydrographical properties of the Neretva channel (eastern Adriatic Sea). Helgol Mar Res 61:267–282
Zervoudaki S, Christou ED, Nielsen TG, Siokou Frangou I, Assimakopoulou G, Giannakourou A, Maar M, Pagou K, Krasakopoulou E, Christaki U, Moraitou Apostolopoulou M (2007) The importance of small-sized copepods in a frontal area of the Aegean Sea. J Plankton Res 29:317–338
Acknowledgements
This work was conducted in the Biodiversity and Aquatic Ecosystems UR/11ES72 Research Unit at the University of Sfax.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
We declare that we have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Rekik, A., Ayadi, H. & Elloumi, J. Spatial and inter-annual variability of proto- and metazooplankton during summer around the Kneiss Islands (Tunisia, Central Mediterranean Sea). Appl Water Sci 8, 99 (2018). https://doi.org/10.1007/s13201-018-0744-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13201-018-0744-4